-
PDF
- Split View
-
Views
-
Cite
Cite
Balakavitha Balaravi Pillai, Bushra Othman, Eugenia Ip, Rare direct embolism of urothelial carcinoma causing acute mesenteric ischaemia during remission, Journal of Surgical Case Reports, Volume 2023, Issue 3, March 2023, rjad129, https://doi.org/10.1093/jscr/rjad129
Close - Share Icon Share
Abstract
Metastatic abdominal carcinomas have been reported in the literature to cause bowel ischaemia. However, these are often associated with diffuse disease or direct invasion of adjacent bowel secondary to high-grade malignancies. There are no reported cases of extensive small bowel wall nor arterial occlusion as a result of metastasis from treated early-stage urothelial carcinoma. We present an octogenarian male patient who was diagnosed with small bowel ischaemia secondary to extensive metastatic urothelial carcinomatous to the small bowel. Thorough consideration of the patient’s clinical presentation and high index of suspicion was required to differentiate this from other causes of ischaemic bowel. The pathophysiology of the disease differentiated the end treatment most suitable for the patient.
INTRODUCTION
Urothelial carcinoma (UC) of the bladder may metastasise to the gastrointestinal tract at a rate of 3–5% and to the peritoneum at 16% in Stage IV disease [1, 2]. Manifestations of metastatic disease as the primary presenting symptom may be unexpected, especially amongst patients in remission for a prolonged period. Hoshi et al. described two patients with incidences of small intestinal metastasis of urothelioma within 6 months from their primary surgical resection [3]. However, reports of extensive metastatic disease to the small bowel and embolism of metastatic disease despite intensive surveillance and remission for over a decade has not been reported.
CASE REPORT
The patient, who was in his early eighties, was admitted to the emergency department with severe abdominal pain and vomiting, on a background of multiple self-limiting episodes of similar pain for which no radiological or endoscopic causes had been identified. His past medical history included therapeutic anticoagulation for bilateral lower limb deep vein thrombosis and cardioembolic stroke. In addition, he was diagnosed with bladder carcinoma in situ (T1 disease) 8 years prior for which he underwent transurethral resection of bladder tumor (TURBT) and intravesical Bacillus Calmette-Guerin therapy. No recurrence had been detected with intensive yearly surveillance cystoscopies and biopsies, including that performed eight months prior to presentation.
On presentation, his physical examination revealed an acute abdomen with generalised peritonism. Computed tomography (CT) angiogram of the abdomen suggested a thrombus in a distal branch of the superior mesenteric artery (SMA) without evidence of bowel compromise (Fig. 1). The preoperative provisional diagnosis for this patient was mesenteric arterial occlusion by arterial thrombosis. Differential diagnoses included mesenteric venous thrombosis, arterial embolism, and non-occlusive mesenteric ischaemia.
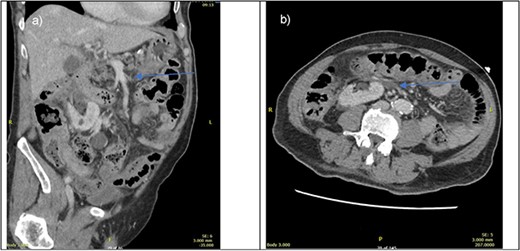
CT portal venous phase (left—sagittal slice, right axial slice) of imaging done upon presentation. Blue arrow pointing towards suspected thrombus causing bowel ischaemia.
Thus, the patient was commenced on therapeutic intravenous heparin infusion and proceeded to a diagnostic laparoscopy. At laparoscopy, all small and large bowels appeared macroscopically normal and viable, and the decision was made for ongoing anticoagulation for treatment of the initial diagnosis of the SMA thrombus without a need for bowel resection. At 36 h following surgery, the patient reported worsening abdominal pain and tachycardia. Repeat CT angiogram revealed resolution of a large component of the SMA thrombus but persistent occlusion with changes, suggestive of ongoing distal small bowel ischaemia.
The patient proceeded to an exploratory laparotomy where no small bowel ischaemia was identified but the distal small bowel felt thickened on palpation with a small pocket of pus on the antimesenteric border, suggestive of impending perforation (Fig. 2). One-hundred cm of thickened small bowel on palpation was resected and a laparostomy was performed. A planned relook laparotomy after 24 hours revealed macroscopically viable small bowel and an anastomosis was performed. Interestingly, histopathology of the resected small bowel revealed extensive deposits of urothelial carcinoma within the mucosa including at the resection margins and the mesenteric arterial arcade, suggesting a widespread embolism of the metastatic disease (Fig. 3).
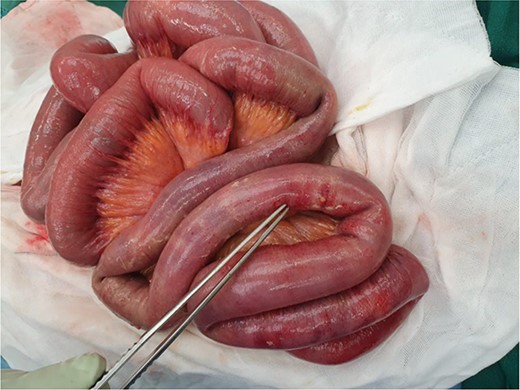
Intraoperative photograph of the visually normal but thickened small bowel on palpation at laparotomy (second surgery). Forceps pointing to an area of pus with pinpoint perforation.
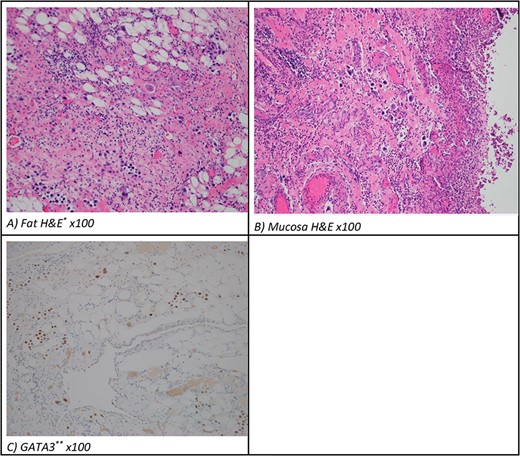
(A) Mesentery and (B) bowel pathology (H&E, original magnification, 100×). (C) Resected metastatic small bowel pathology (GAT3 ×100) typical for either urothelioma or breast carcinoma. *H&E, hematoxylin and eosin. **GATA3 is a transcription factor important in the differentiation of breast epithelia, urothelial and subsets of T-lymphocytes.
Given the histopathology findings and the patient’s postoperative poor respiratory function, treatment futility was discussed with the family with eventual palliative care plans commenced through a multi-specialty discussion among Oncologist, Intensivist, Vascular and General Surgeons. The patient died ten days following his initial operation.
DISCUSSION
Urothelial carcinoma, formerly known as transitional cell carcinoma, is the most common form of bladder cancer and has a high multiplicity rate [4]. High-grade T1 (HGT1) bladder cancer is associated with a high recurrence rate and risk of progression thereby necessitating stringent endoscopic and radiological surveillance [5, 6]. Lymphatic spread of the tumor primarily occurs to locoregional inguinal and common iliac nodes followed by spread to paraaortic lymph nodes. Distant spread via the haematogenous route commonly affects the lung, bone, liver and adrenal glands [7]. The gastrointestinal tract and mesentery are rare sites of metastasis, and only three cases of manifestations of metastasis to the small intestine have been previously reported in patients with terminal stage of the disease [8, 9]. The presentation of ischaemic gut secondary to isolated small bowel mucosal urothelial metastases in the absence of bladder recurrence has yet to be reported.
Mesenteric ischemia accounts for 1% of patients with acute abdomen and approaches a mortality rate of approximately 60% [10]. Patients often are diagnosed with a variety of symptoms, but the classical description is that of pain out of proportion to physical examination. Embolic occlusion of the SMA occurs in 40–50% of cases, whereas thrombotic occlusion occurs in 20–30% of cases [11]. Risk factors include advanced age and intraabdominal malignancy. For these patient’s thrombolysis with endovascular angioplasty, stenting or surgical revascularisation is the main stay of treatment [12, 13]. Limited literature discusses the survival benefit of treatment of patients with arterial occlusion directly by tumor deposits. A case series of 20 patients with malignancy and acute limb ischaemia have a survival rate of 50% at 3 months and 17% at 1 year from the time of presentation of arterial thrombosis [14].
In this case, there was no prior history of abdominal surgery since the initial diagnosis of UC many years prior, nor was there any bladder perforation during cystoscopy or the initial TURBT. The hypothesis of tumor spillage or seeding from prior surgery leading to metastatic disease, therefore, seems less likely. During the initial laparoscopy, instrument handling via bowel graspers revealed macroscopically normal bowel. The relook laparoscopy revealed no overt bowel ischaemia or necrosis, but a small area of bowel appeared abnormal. The utility of manual handling of the bowel in laparoscopy and the reduced direct tactile component of laparoscopic graspers led to the suspicion of an underlying disease process within the internal layers of the bowel wall. It became evident during the laparotomy that the distal ileum was thickened and abnormal and required resection.
In conclusion, the learning points are that an unusual case of delayed urothelial carcinoma metastasis to the small bowel and directly occluding the arterial mesentery can be expected despite previous treatment of early-stage carcinoma, even during the period of surveillance cystoscopy and biopsy. Furthermore, tactile examination of the bowel may assist in the differentiation of causes of any small bowel ischaemia.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



