-
PDF
- Split View
-
Views
-
Cite
Cite
Olufemi I Idowu, Hammed Oshola, Edobor F Emiogun, An unexpected diagnosis of malignant supratentorial intraparenchymal cystic meningioma mimicking high-grade glioma: case report and literature review, Journal of Surgical Case Reports, Volume 2023, Issue 2, February 2023, rjad062, https://doi.org/10.1093/jscr/rjad062
Close - Share Icon Share
Abstract
Meningiomas are generally dura-based extra axial tumours without cystic components, whereas high-grade gliomas are intra-axial with or without cystic component. This case describes an adult female who presented with clinical and radiological features suggestive of a high-grade astrocytoma; however, histology diagnosis was papillary meningioma (World Health Organization Grade III). A 58-year-old female presented with a 4-month history of recurrent generalized tonic–clonic seizures and a 1-week history of altered sensorium. Her Glasgow Coma Scale Score was 10. Magnetic resonance image revealed a large intra-axial heterogeneous solid mass with multiple cystic areas in the right parietal lobe. She subsequently had craniotomy and tumour excision and the histologic diagnosis was papillary meningioma (WHO Grade III). Rarely, meningioma can present as an intra-axial tumour and may mimic other lesions like high-grade astrocytoma.
INTRODUCTION
Meningioma is one of the most common primary intracranial tumours. It accounts for ~20 and 38% of intracranial tumours in males and females, respectively [1]. They commonly present as benign, slow growing and highly vascular spherical or oval masses with a dura attachment. However, occurrence of meningiomas without a dura attachment is rare. According to their locations, meningiomas without dura attachment can be classified into five main types, namely, intraparenchymal (subcortical), pineal region, deep Sylvain fissure, intraventricular and others [2].
Because of the rarity and location, intraparenchymal meningioma (IPM) is often misdiagnosed preoperatively as gliomas, cavernous angioma, malignant lymphoma or metastatic tumours. The preoperative misdiagnosis can also occur if there are cystic components within the tumour [3].
CASE REPORT
A 58-year-old right-handed female presented to our department with a 4-month history of recurrent generalized tonic–clonic seizures and a 1-week history of altered sensorium. One day prior to presentation at the hospital, the patient had become unresponsive to calls. There was an antecedent history of throbbing headaches, which was attributed to post-traumatic concussion syndrome following treatment for a traumatic brain injury, 8 months prior to presentation.
Examination at presentation revealed a middle-aged woman. Her Glasgow Coma Scale Score was 10. She had left facioparesis, upper motor neuron type and left spastic hemiparesis. The power grade on the left was Grade 1.
Cranial magnetic resonance image (MRI) revealed a large heterogeneous solid mass with multiple cystic areas in the right parietal lobe; this was associated with marked mass effect (Fig. 1A and B). The total brain lesion measured 7.2 × 6.0 × 7.3 cm, with the solid lateral component measuring 2.9 × 2.8 × 4.1 cm. There was heterogeneous contrast enhancement with extensive perilesional oedema. Magnetic resonance spectroscopy revealed decrease N-acetyl aspartate, creatinine and myoinositol and markedly increased lactate (Fig. 1C).
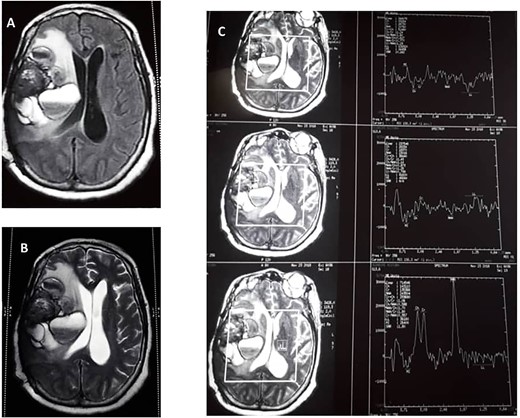
(A) Preoperative T1 weight. (B) T2-weighted MRIs. (C) Preoperative magnetic resonance spectroscopy.
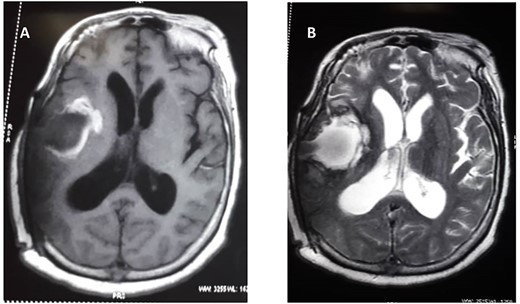
She subsequently had right frontotemporal craniotomy and tumour excision. Intraoperative findings were those of a mixed consistency mass, which was firm, gritty with areas of necrosis and brown coloured cystic components. Histopathological examination of the tumour revealed neoplastic papillary structures infiltrating a dense fibro-collagenous matrix. The neoplastic papillae showed discohesive cells lining delicate fibrovascular cores; the cells exhibited marked nuclear pleomorphism with hyperchromatic and vesicular nuclei. Some abnormal mitotic figures were also seen and there were no residual neural tissues present (Fig. 2A–C). The overall histopathological features were consistent with papillary meningioma (World Health Organization Grade III). The MRI at 3 months post-operative showed gross total tumour resection with an area of cystic cavitation because of encephalomalacia from the previous tumour and surgery (Fig. 3A and B). She subsequently had adjuvant radiotherapy and was lost to follow-up.
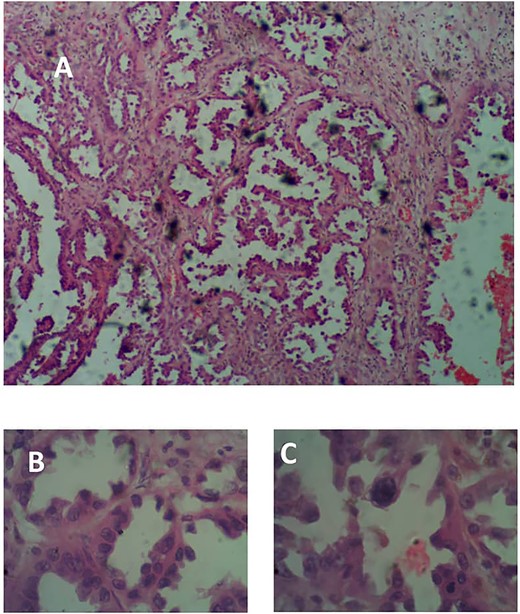
(A) Photomicrograph showing arborizing neoplastic papillae H&E × 40. (B) H&E × 100. (C) Showing high-grade neoplastic cells lining delicate fibrovascular cores, H&E × 400.
DISCUSSION
Adult dura-based meningiomas tend to occur more frequently in females, with overall female-to-male ratio of 2.6:1 [4]. When dura-based meningiomas occur, they usually present in adulthood. This is at variance to IPM. In a meta-analysis of 39 different case reports of IPM by Ohba et al, who reported that two-third of the cases were in patients younger than 20 years old. Considering the proportion of meningiomas that occur in children, it can be said that IPM tend to develop in children, with most of the IPM (87.2%) occurring in the cerebrum [5]. Of the five patients with malignant IPM reported in the literature, two were <18 years of age and two were males (Table 1) [6–10].
| Author(s) . | Gender . | Age (years) . | Clinical feature(s) . | Tumour location . | Pathology . |
|---|---|---|---|---|---|
| Morimoto et al. [6] | Female | 17 | Seizure | Parietal | Malignant |
| Kim et al. [7] | Female | 41 | Seizure | Frontal | Anaplastic |
| Nayil et al. [8] | Male | 3 | Headaches and vomiting | Frontal | Anaplastic |
| Dutta et al. [9] | Female | 36 | Headaches | Temporal | Rhabdoid |
| Jiang et al. [10] | Male | 23 | Upper limb weakness | Brainstem | Papillary |
| Idowu et al. (2020) | Female | 58 | Seizures, headaches and altered sensorium | Frontotemporal | Papillary |
| Author(s) . | Gender . | Age (years) . | Clinical feature(s) . | Tumour location . | Pathology . |
|---|---|---|---|---|---|
| Morimoto et al. [6] | Female | 17 | Seizure | Parietal | Malignant |
| Kim et al. [7] | Female | 41 | Seizure | Frontal | Anaplastic |
| Nayil et al. [8] | Male | 3 | Headaches and vomiting | Frontal | Anaplastic |
| Dutta et al. [9] | Female | 36 | Headaches | Temporal | Rhabdoid |
| Jiang et al. [10] | Male | 23 | Upper limb weakness | Brainstem | Papillary |
| Idowu et al. (2020) | Female | 58 | Seizures, headaches and altered sensorium | Frontotemporal | Papillary |
| Author(s) . | Gender . | Age (years) . | Clinical feature(s) . | Tumour location . | Pathology . |
|---|---|---|---|---|---|
| Morimoto et al. [6] | Female | 17 | Seizure | Parietal | Malignant |
| Kim et al. [7] | Female | 41 | Seizure | Frontal | Anaplastic |
| Nayil et al. [8] | Male | 3 | Headaches and vomiting | Frontal | Anaplastic |
| Dutta et al. [9] | Female | 36 | Headaches | Temporal | Rhabdoid |
| Jiang et al. [10] | Male | 23 | Upper limb weakness | Brainstem | Papillary |
| Idowu et al. (2020) | Female | 58 | Seizures, headaches and altered sensorium | Frontotemporal | Papillary |
| Author(s) . | Gender . | Age (years) . | Clinical feature(s) . | Tumour location . | Pathology . |
|---|---|---|---|---|---|
| Morimoto et al. [6] | Female | 17 | Seizure | Parietal | Malignant |
| Kim et al. [7] | Female | 41 | Seizure | Frontal | Anaplastic |
| Nayil et al. [8] | Male | 3 | Headaches and vomiting | Frontal | Anaplastic |
| Dutta et al. [9] | Female | 36 | Headaches | Temporal | Rhabdoid |
| Jiang et al. [10] | Male | 23 | Upper limb weakness | Brainstem | Papillary |
| Idowu et al. (2020) | Female | 58 | Seizures, headaches and altered sensorium | Frontotemporal | Papillary |
Meningiomas rarely have a cystic component except for the high-grade variety. The neuroimage in patients with IPM is non-specific and thus most cases are misdiagnosed preoperatively. Compared with dura-based meningiomas, IPM tend to contain cystic components. High-grade meningiomas also tend to be cystic. This is similar to the findings in our reported case. The cystic component is one of the characteristic features of IPM. However, the presence of an associated cyst in IPM may make it difficult to distinguish it from a primary intra-axial glial neoplasm. Likewise, the presence of peritumoral oedema may also make the diagnosis of IPM difficult.
On MRI images, IPM appears as hypointense solid masses on T1WI images. On T2WI images, hypointensity, hyperintensity or mixed intensity signals may be observed. The lesion usually enhances significantly in a homogeneous or heterogeneous pattern; however, some tumours do not demonstrate enhancement [3].
CONCLUSION
Since the treatment strategy and operative techniques used to manage meningiomas differ from that used for gliomas, cavernous angioma, malignant lymphoma or metastatic tumours, it is important to keep in mind the possibility of malignant IPM in mixed consistency intraparenchymal tumour.
ETHICAL APPROVAL AND CONSENT
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
DATA AVAILABILITY
Not applicable.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
AUTHORS’ CONTRIBUTIONS
O.I.I.—research project conceptualization and design; H.O.—research project execution; E.F.E.—performed histological examination of the tumour specimen and edited the manuscript.



