-
PDF
- Split View
-
Views
-
Cite
Cite
Raelina S Howell, Shrey Shah, Saila Khan, Collin E M Brathwaite, Patrizio Petrone, Jun Levine, Kwashiorkor after gastric bypass, Journal of Surgical Case Reports, Volume 2023, Issue 2, February 2023, rjad030, https://doi.org/10.1093/jscr/rjad030
Close - Share Icon Share
Abstract
Gastric bypass has grown in popularity in recent years due to its high efficacy in achieving long-term weight loss in patients with morbid obesity. Gastric bypass has been described to further exacerbate baseline nutritional deficiencies due to reduced gastric capacity and malabsorption. In rare cases, when protein deficiency is severe, Kwashiorkor disease may arise. The incidence of Kwashiorkor specifically following gastric bypass is rare, with an incidence of 4.7%. We report a case of a female patient who underwent a gastric bypass and subsequently developed Kwashiorkor. Physicians’ suspicion of index for Kwashiorkor should be high for patients presenting with signs or symptoms of severe malnutrition following weight-loss procedures.
INTRODUCTION
Gastric bypass has grown in popularity in recent years due to its high efficacy in achieving long-term weight loss in patients with morbid obesity. Gastric bypass has been described to further exacerbate baseline nutritional deficiencies due to reduced gastric capacity and malabsorption [1]. In rare cases, when the protein deficiency is severe, Kwashiorkor disease may arise [2–4], which is characterized by pitting edema, abdominal distention and enlarged liver with fatty infiltrate. Although more common in resource-poor countries, Kwashiorkor is often secondary to non-dietary causes in developed countries, including bariatric surgery, chronic alcoholism, kidney disease, severe burns and anorexia nervosa [5]. In some cases of gastric bypass surgery, secondary Kwashiorkor can develop after self-prescribed diet modifications in order to achieve a greater weight loss. Patients may substitute protein with carbohydrate-rich, high-calorie alternatives without understanding the consequences [4]. We report a case of Kwashiorkor disease, an important but rare sequelae of gastric bypass surgery, that was diagnosed 12 years after gastric bypass.
CASE REPORT
A 44-year-old-female with a surgical history notable for gastric bypass 12 years prior presented to the emergency department (ED) with a chief complaint of intermittent diarrhea, weight loss and nausea controlled by ondansetron. The patient had been admitted 8 months prior to presentation for abdominal pain and constipation that resolved with PO medications; however, she reported diarrhea since that time. The patient also reported that she was constantly hungry and eating high protein diet. She also complained of swelling of the bilateral arms, legs and abdomen for which her furosemide was increased on an outpatient basis. In the ED, her labs were significant for total protein = 4.2 gm/dl, albumin = 2.1 gm/dl, pre-albumin = 12.8 mg/dl, iron = 12 mcg/dl and calcium = 7.2 mg/dl. During the hospital course, the albumin was noted to decrease to 1.7 gm/dl. An abdominal ultrasound was performed and was only significant for a small amount of ascites. Gastroenterology was consulted and she was recommended with upper endoscopy, colonoscopy and a small bowel series in addition to total parenteral nutrition (TPN). Endoscopies were unremarkable and the small bowel series revealed a granular appearance of the jejunal mucosa with irregular thickening of the most proximal jejunal loop (Fig. 1). The patient was tolerating regular diet with persistent diarrhea; therefore, TPN was continued upon discharge to a rehabilitation facility in addition to her diet.
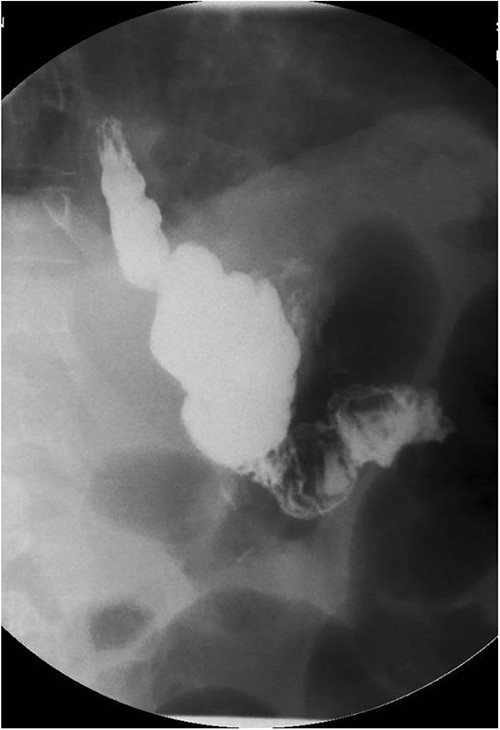
Small bowel series showing normal contrast transit from esophagus to stomach to small bowel loop.
Upon follow-up 2 weeks later, the patient was found to have continued metabolic derrangements (hemoglobin = 6.6 g/dl, total protein = 5.0 gm/dl, albumin = 2.8 gm/dl and calcium = 7.8 mg/dl). Admission was recommended and the patient reported fatigue, shortness of breath, abdominal pain, diarrhea and bilateral lower extremity swelling. Initial labs were significant for hemoglobin 6.8 g/dl for which she was given one unit of packed red blood cells. Capsule video endoscopy was performed and it showed focal, small bowel ulceration with a low suspicion for inflammatory bowel disease (IBD). Empiric treatment with pancrelipase, mesalamine and levofloxacin were started for possible pancreatic insufficiency and IBD, respectively. However, the patient left the floor, without signing, against medical advice and we were unable to pursue further workup as she never returned for follow-up.
DISCUSSION
Kwashiorkor, also known as protein-calorie malnutrition, is a nutritional disorder that was recognized as a public health crisis by the World Health Organization in the 1950s [6]. Infants and children up to age 5 are the most commonly affected in the under-developed regions, with two forms of severe acute malnutrition which are distinguished as Kwashiorkor and marasmus [7]. Children with Kwashiorkor have nutritional edema and metabolic disturbances, including hypoalbuminemia and hepatic steatosis due to protein deficiency despite adequate energy intake [7]. By contrast, marasmus is characterized by severe wasting due to inadequate energy intake of all forms, including protein [7]. Kwashiorkor is not prevalent in regions with sufficient food resources, but isolated cases can occur in the elderly, neglected or abused children, or iatrogenically. The occurrence of metabolic derangements following weight-loss operations has reduced due to improvements in surgical technique, nutritional supplementation and physician follow-up over the years [8]. Patients who undergo gastric bypass are at risk of developing malnutrition due to surgical exclusion of a portion of the digestive tract. As in our case, the patient reported eating a large amount of high protein diet but still developed Kwashiorkor, supporting malabsorption pathophysiology. Post-operative nutritional deficiencies are usually corrected with the appropriate supplementation or TPN without progression [9]. The incidence of Kwashiorkor specifically following gastric bypass is rare, with one report of 11 patients out of 236 over a 68-month period finding an incidence of 4.7% [8].
The pathogenesis of Kwashiorkor has remained elusive even after past 60 years of research, and there have been many ideas, including inadequate dietary protein, the leaky gut syndrome (compromised gut epithelial barrier) and intestinal inflammation [10]. The hallmark signs of Kwashiorkor are protein malnutrition and bilateral lower extremity swelling [6]. Serum albumin protein depletion is characteristic of Kwashiorkor and can be severe. Peripheral edema occurs as a result of hypoalbuminemia, leading to intravascular fluid extravasation. Antidiuretic hormone and renin levels increase in response to the hypovolemia, further exacerbating the edema. Clinical findings include peripheral pitting edema, irritability, anorexia, diarrhea, hair loss, abdominal distention and ‘flaky paint’ dermatitis in areas of pressure [2, 6]. The histopathological findings are not pathognomonic and include parakeratosis, balloon in degeneration of keratinocytes in a band-like formation in the superficial epidermis and edema and a lymphocytic perivascular infiltrate in the upper dermis [2]. Treatment consists of a slow increase in calories which is followed by an increase in protein in diet and life-long nutritional follow-up. Delay in treatment is associated with high rates of morbidity and mortality secondary to infection, hemodynamic instability or malabsorption syndrome [2–4]. Physicians’ suspicion of index for Kwashiorkor should be high for patients presenting with signs or symptoms of severe malnutrition following weight-loss procedures.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
DATA AVAILABILITY
Data is not publicly available.



