-
PDF
- Split View
-
Views
-
Cite
Cite
Ling Dong, Chunxia Huang, Benli Jia, Ye Zhang, Qi Xue, Anesthesia and perioperative management of bariatric surgery in a patient with BMI over 70 kg/m2: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 12, December 2023, rjad650, https://doi.org/10.1093/jscr/rjad650
Close - Share Icon Share
Abstract
The anesthesia protocol of bariatric surgery has not been standardized in detail. This report introduced an optimized anesthesia strategy for a severely obese male patient for laparoscopic sleeve gastrectomy and a continuous follow-up for 12 months. A 34-year-old male patient was admitted for super-super-obesity with obstructive sleep apnea-hypopnea syndrome. Based on the results of the examination before laparoscopic sleeve gastrectomy, he received an efficient preoperative exercise guidance for pulmonary function promotion and diet restriction. Multiple analgesia scheme consisted of regional nerve block and analgesics cocktail. He lost 103 kg without any complications till postoperative 12 months. His mental characteristics were also improved gradually. This case presented a superior composition in perioperative anesthesia management for laparoscopic sleeve gastrectomy. To provide a reference for promoting the implementation of enhanced recovery after surgery protocols in bariatric surgery.
Introduction
Enhanced recovery after surgery (ERAS) programs have been shown to improve postoperative recovery and reduce hospital stay, complications, readmission, and cost in multiple types of surgery [1]. In the updated guidelines for perioperative care in bariatric surgery, it provided recommendations covering each ERAS item involving preoperative optimization, standardized anesthesia, and postoperative nutritional care [2]. In particular, it strongly recommends multi-aspect perioperative management, including preoperative exercise and multimodal, opioid-preserving analgesic approaches. Regarding the coexistence of obstructive sleep apnea-hypopnea syndrome and high pain sensitivity, as well as the tendency of persisted long-term severe pain [3], appropriate anesthesia strategy challenged every anesthesiologist. Hereby, we reported, from the perspective of anesthesiologists, the entire process of perioperative managing a patient with a BMI over 70 kg/m2 who underwent laparoscopic sleeve gastrectomy (LSG), and the continuous follow-up in postoperative 12 months.
Case presentation
A 34-year-old male patient was admitted to our hospital on 12 May 2020 because of super-super-obesity (height 171 cm, body weight 225 kg, BMI 76.95 kg/m2) with obstructive sleep apnea-hypopnea syndrome.
After the admission, Blood gas analysis showed that PaO2 and PaCO2 were 68.4 and 59.6 mmHg, respectively. We asked him to blow 10 new balloons four times a day (each balloon blows five times), to exercise and diet. Then, after the completion of relevant examinations and the exclusion of contraindications, LSG was settled on 25 May 2020.
In the pre-anesthesia unit, ultrasound-guided transversus abdominis plane (TAP) block (Sonosite Micromaxx, Bothell, WA, USA) was performed bilaterally. Ropivacaine (0.33%, 30 mL/injection) with dexmedetomidine (1 μg/kg) was injected in the fascial plane under the costal margin. And the arterial blood analysis presented PaO2 75 mmHg, PaCO2 42 mmHg, and fasting blood glucose 4.2 mM. Then he has received a bolus infusion of dexmedetomidine 2 μg/kg/h for 10 min at a semi-upright position. Intravenous anesthesia induction consisted of midazolam 0.025 mg/kg, propofol 1.5 mg/kg, cisatracurium 0.2 mg/kg, and remifentanil 2 μg/kg. Anesthesia maintenance was achieved by sevoflurane inhalation accompanied by a continuous infusion with remifentanil 5 μg/kg/h, cisatracurium 0.1 mg/kg/h, and dexmedetomidine 0.4 μg/kg/h. The dosages of all intravenous anesthetics were calculated by IBW [ideal body weight (kg) = height (mm)-100]. To alleviate the visceral pain, parecoxib 40 mg and nalbuphine 10 mg were used before the surgery initiation. Thirty minutes before the end of the surgery, 3 mg granisetron, as prophylaxis against PONV was administered. Systemic postoperative analgesia was applied by patients-controlled intravenous analgesia (sufentanil 2 μg/kg, dexmedetomidine 2 μg/kg, and granisetron 3 mg) for 48 h. An initial bolus of 2 mL was programmed to deliver followed by 0.8 mL bolus (lockout interval = 15 min, infusion rate = 2 mL/h).
Half an hour following surgery initiation, SPO2 was dropped to 95%. We performed a manual recruitment maneuver, then SPO2 was maintained at 100% till the end of surgery. Arterial blood analysis showed PaO2 186 mmHg, PaCO2 43 mmHg, and glucose 7.3 mM immediately after gastrectomy. Surgical procedures were completed within 75 min by the skilled surgeon team. During the entire surgery, 6 mg ephedrine and 244.3 μg phenylephrine were used to maintain arterial blood pressure. Total anesthetic consumption was propofol 190.3 mg, remifentanil 745.5 μg, cisatracurium 12.27 mg, and dexmedetomidine 70.36 μg. He was discharged 3 days later without postoperative pain, nausea, or vomiting experience.
During the 12-month follow-up period, there was no recorded complication. He reported a gradual physical improvement. At postoperative 2, 6, and 12 months, his body weight losses were 26.1%, 36.7%, and 45.8% respectively. BMI was also dropped to 39.8 kg/m2 at postoperative 12 months (Fig. 1). Results of laboratory tests were fluctuated at postoperative 2 months but returned to normal at postoperative 6 and 12 months (Table 1). In addition, we used the Hamilton anxiety scale (HAMA), Hamilton depression scale (HAMD), and Toronto alexithymia scale (TAS) to assess his mental health. The scores were gradually declined, which suggested the improvement of mental status (Fig. 2).
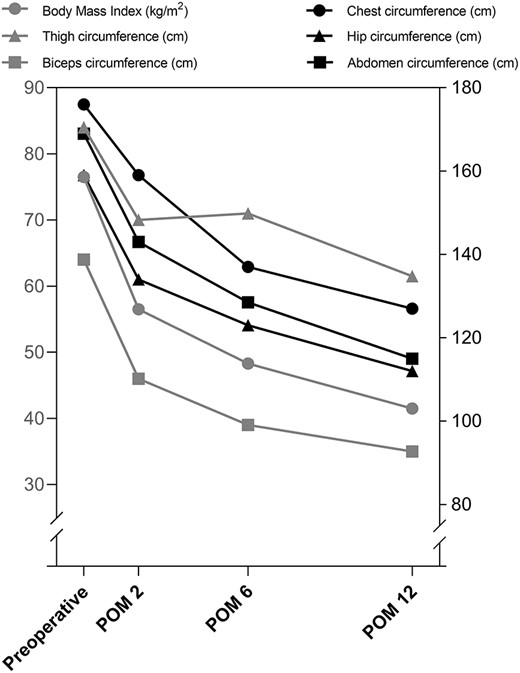
Changes in characteristics over Time. POM, postoperative month.
| Variable . | Preoperative . | POM2 . | POM 6 . | POM 12 . | Reference . |
|---|---|---|---|---|---|
| Alanine aminotransferase (U/L) | 28 | 23.2 | 40 | 14 | 9–50 |
| Aspartate aminotransferase (U/L) | 37 | 34 | 25 | 17 | 15–40 |
| Total Bilirubin (mol/L) | 29 | 40.5 | 10.5 | 16.6 | 0.0–26.0 |
| Direct bilirubin (μmol/L) | 6.6 | 10.3 | 1.7 | 2.8 | 0.0–4.0 |
| Indirect bilirubin (μmol/L) | 22.4 | 30.2 | 8.8 | 13.8 | 0.0–22.0 |
| Blood urea nitrogen (mmol/L) | 5.04 | 3.1 | 6.42 | 4.67 | 3.10–8.00 |
| Creatinine (μmol/L) | 42 | 71 | 87 | 79 | 57–97 |
| Estimated glomerular filtration rate [ml/(min.l.)] | 266.23 | / | 114.19 | 126.89 | >90.00 |
| Uric Acid (μmol/L) | 569 | 833 | 506 | 458 | 208–428 |
| Fasting plasma glucose (mmol/L) | 4.04 | 5.54 | 5.5 | 5.12 | 3.90–6.10 |
| Cholesterol (mmol/L) | 4.06 | 6.79 | 3.85 | 3.75 | 2.86–5.98 |
| Triglycerides (mmol/L) | 0.85 | 2.33 | 2.33 | 1.4 | 0.56–1.70 |
| High-density lipoprotein cholesterol (mmol/L) | 0.92 | 0.87 | 0.97 | 1.06 | 0.94–2.00 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.79 | 4.58 | 2.46 | 2.51 | 0.00–2.60 |
| Variable . | Preoperative . | POM2 . | POM 6 . | POM 12 . | Reference . |
|---|---|---|---|---|---|
| Alanine aminotransferase (U/L) | 28 | 23.2 | 40 | 14 | 9–50 |
| Aspartate aminotransferase (U/L) | 37 | 34 | 25 | 17 | 15–40 |
| Total Bilirubin (mol/L) | 29 | 40.5 | 10.5 | 16.6 | 0.0–26.0 |
| Direct bilirubin (μmol/L) | 6.6 | 10.3 | 1.7 | 2.8 | 0.0–4.0 |
| Indirect bilirubin (μmol/L) | 22.4 | 30.2 | 8.8 | 13.8 | 0.0–22.0 |
| Blood urea nitrogen (mmol/L) | 5.04 | 3.1 | 6.42 | 4.67 | 3.10–8.00 |
| Creatinine (μmol/L) | 42 | 71 | 87 | 79 | 57–97 |
| Estimated glomerular filtration rate [ml/(min.l.)] | 266.23 | / | 114.19 | 126.89 | >90.00 |
| Uric Acid (μmol/L) | 569 | 833 | 506 | 458 | 208–428 |
| Fasting plasma glucose (mmol/L) | 4.04 | 5.54 | 5.5 | 5.12 | 3.90–6.10 |
| Cholesterol (mmol/L) | 4.06 | 6.79 | 3.85 | 3.75 | 2.86–5.98 |
| Triglycerides (mmol/L) | 0.85 | 2.33 | 2.33 | 1.4 | 0.56–1.70 |
| High-density lipoprotein cholesterol (mmol/L) | 0.92 | 0.87 | 0.97 | 1.06 | 0.94–2.00 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.79 | 4.58 | 2.46 | 2.51 | 0.00–2.60 |
POM, postoperative month.
| Variable . | Preoperative . | POM2 . | POM 6 . | POM 12 . | Reference . |
|---|---|---|---|---|---|
| Alanine aminotransferase (U/L) | 28 | 23.2 | 40 | 14 | 9–50 |
| Aspartate aminotransferase (U/L) | 37 | 34 | 25 | 17 | 15–40 |
| Total Bilirubin (mol/L) | 29 | 40.5 | 10.5 | 16.6 | 0.0–26.0 |
| Direct bilirubin (μmol/L) | 6.6 | 10.3 | 1.7 | 2.8 | 0.0–4.0 |
| Indirect bilirubin (μmol/L) | 22.4 | 30.2 | 8.8 | 13.8 | 0.0–22.0 |
| Blood urea nitrogen (mmol/L) | 5.04 | 3.1 | 6.42 | 4.67 | 3.10–8.00 |
| Creatinine (μmol/L) | 42 | 71 | 87 | 79 | 57–97 |
| Estimated glomerular filtration rate [ml/(min.l.)] | 266.23 | / | 114.19 | 126.89 | >90.00 |
| Uric Acid (μmol/L) | 569 | 833 | 506 | 458 | 208–428 |
| Fasting plasma glucose (mmol/L) | 4.04 | 5.54 | 5.5 | 5.12 | 3.90–6.10 |
| Cholesterol (mmol/L) | 4.06 | 6.79 | 3.85 | 3.75 | 2.86–5.98 |
| Triglycerides (mmol/L) | 0.85 | 2.33 | 2.33 | 1.4 | 0.56–1.70 |
| High-density lipoprotein cholesterol (mmol/L) | 0.92 | 0.87 | 0.97 | 1.06 | 0.94–2.00 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.79 | 4.58 | 2.46 | 2.51 | 0.00–2.60 |
| Variable . | Preoperative . | POM2 . | POM 6 . | POM 12 . | Reference . |
|---|---|---|---|---|---|
| Alanine aminotransferase (U/L) | 28 | 23.2 | 40 | 14 | 9–50 |
| Aspartate aminotransferase (U/L) | 37 | 34 | 25 | 17 | 15–40 |
| Total Bilirubin (mol/L) | 29 | 40.5 | 10.5 | 16.6 | 0.0–26.0 |
| Direct bilirubin (μmol/L) | 6.6 | 10.3 | 1.7 | 2.8 | 0.0–4.0 |
| Indirect bilirubin (μmol/L) | 22.4 | 30.2 | 8.8 | 13.8 | 0.0–22.0 |
| Blood urea nitrogen (mmol/L) | 5.04 | 3.1 | 6.42 | 4.67 | 3.10–8.00 |
| Creatinine (μmol/L) | 42 | 71 | 87 | 79 | 57–97 |
| Estimated glomerular filtration rate [ml/(min.l.)] | 266.23 | / | 114.19 | 126.89 | >90.00 |
| Uric Acid (μmol/L) | 569 | 833 | 506 | 458 | 208–428 |
| Fasting plasma glucose (mmol/L) | 4.04 | 5.54 | 5.5 | 5.12 | 3.90–6.10 |
| Cholesterol (mmol/L) | 4.06 | 6.79 | 3.85 | 3.75 | 2.86–5.98 |
| Triglycerides (mmol/L) | 0.85 | 2.33 | 2.33 | 1.4 | 0.56–1.70 |
| High-density lipoprotein cholesterol (mmol/L) | 0.92 | 0.87 | 0.97 | 1.06 | 0.94–2.00 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.79 | 4.58 | 2.46 | 2.51 | 0.00–2.60 |
POM, postoperative month.
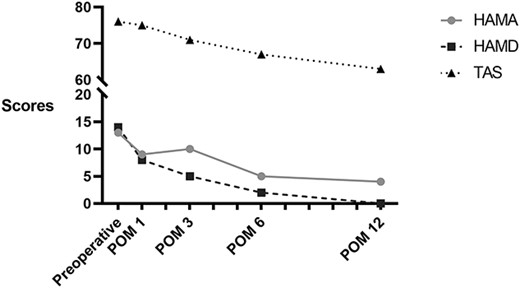
Discussion and conclusions
For bariatric surgery, recent evidence has shown that ERAS protocols successfully improve analgesia, reduce opiate consumption, shorten the length of hospital stay, and lower costs [4, 5]. To avoid postoperative pulmonary complications, such as atelectasis, we suggested the patient perform an easy practice for pulmonary function exercise before surgery. This may contribute to the increase in PaO2 and reduction in PaCO2.
In this case, the patient reported no postoperative pain in the incision and viscera, neither did postoperative nausea and vomiting. This may be related to multimodal analgesia management, including multimode of analgesics, ultrasound-guided TAP block, and perioperative administration of dexmedetomidine. Parecoxib and nalbuphine can effectively improve the outcome of analgesia [6, 7]. The adjunct with TAP block in bariatric surgery was demonstrated to improve the postoperative analgesia [8, 9], and remained effective till 48 h after the block [10]. Compared with general anesthesia alone, it was reported that TAP block successfully increased patients’ satisfaction with recovery following LSG [11]. It is also evidence that the perioperative administration of dexmedetomidine could reduce opioid use and facilitate recovery [12, 13].
During the 12-month follow-up, the patient lost 103 kg (45.8% of his body weight) without any complications. Simultaneously, a gradual improvement of mental health was also achieved by continuous evaluation with HAMA, HAMD, and TASs. Particularly, at postoperative 2–3 months, the patient experienced cliff descent of his body weight, but the score of HAMA was similar to that at postoperative 1 month. He paid more attention on the acceptance of physical changes and nutrition guidance. It has been emphasized that the management of perioperative psychological health is as important as physical health. And psychological counseling could not be limited to the hospitalization phase, long-term postoperative assessment is also critical for patients’ recovery.
Throughout the ERAS protocol, each discipline has a different focus. For anesthesia, it is essential to stabilize intraoperative hemodynamics and to provide efficient postoperative analgesia. Moreover, anesthesiologists also play an important role in preoperative risk assessment and optimization, and postoperative management [14]. With the expanding popularity of bariatric surgery, to promote the application of ERAS for bariatric surgery, interdisciplinary communication and corporation should be strengthened in detail.
Conflict of interest statement
None declared.
Funding
None declared.
References
- obesity
- body mass index procedure
- analgesics
- diet
- apnea
- dexmedetomidine
- follow-up
- perioperative care
- pain management
- analgesia (pain absence)
- sleep
- pulmonary function
- bariatric surgery
- sleeve gastrectomy, laparoscopic
- transversus abdominis plane block
- slow shallow breathing
- enhanced recovery after surgery
- preoperative exercise



