-
PDF
- Split View
-
Views
-
Cite
Cite
Andinet Beyene, Badhaasaa Bayissa, Mezgebu Atalele, Hamza Umer, Addisu Alemu, Agumasie Semahegn, Pneumatosis cystoids intestinalis in a patient with small bowel obstruction: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 11, November 2023, rjad612, https://doi.org/10.1093/jscr/rjad612
Close - Share Icon Share
Abstract
Pneumatosis cystoides intestinalis is a rare and usually benign condition in which multiple thin-walled cysts develop in the submucosa or subserosa of the gastrointestinal tract. While usually asymptomatic, severe cases can result in pneumoperitoneum, which can be managed surgically or medically depending on circumstances. A 35-year-old male patient presented with signs and symptoms of intestinal obstruction. Then the patient was diagnosed with pneumatosis cystoides intestinalis. The patient underwent surgery, and antibiotic treatment, and was discharged improved with no incident. Pneumatosis cystoides intestinalis is a surgical condition that resembles other life-threatening top surgical emergencies and affects clinicians’ decisions on diagnosis and treatment plans substantially, mainly in low-income countries. So, surgeons have to consider such kind of conditions and avoid the costs and morbidities associated with unnecessary bowel resection or surgery.
Introduction
Pneumatosis cystoides intestinalis (PCI) is a rare condition characterized by multiple intramural gas cysts in the gastrointestinal tract [1–4]. The most common symptoms of PCI are non-specific discomforts, diarrhea, abdominal pain, passage of mucus per rectum, vomiting, constipation, bloating, weight loss, excessive flatulence, and/or rectal bleeding [3, 5]. The co-existence of symptoms of PCI with other manifestations, such as intestinal pseudo-obstruction and/or overgrowth of bacteria, complicates the clinical diagnosis of PCI [3]. Distinguishing between PCI and other life-threatening surgical conditions like pneumatosis intestinalis [6], PCI low incidence, lack of specificity in clinical manifestations, endoscopic findings, often challenges clinicians and misdiagnosed as intestinal polyps, tumors, inflammatory bowel disease, or other conditions [4].
It is a transient, thin cyst containing nitrogen gas in the submucosa and subserosa of the ileum. It is due to hyperperistalsis and increased intraluminal pressure, which forces N2 through the layers of the bowel [5]. However, the etiology of the gas production within the submucosa or the subserosa of the gastrointestinal tract is still unknown [7, 8]. The rupture of the cysts can lead to pneumoperitoneum which can simulate a surgical emergency. The diagnosis of PCI is usually made by colonoscopy, histology, or radiologic findings [4, 7, 9], computed tomography, ultrasonography have demonstrated benefit in patients with multiple nodular projections in the colon [4, 7]. The diagnosis of PCI can be confirmed by cyst collapse after puncturing with a fine needle [7]. Treatment options include bowel rest, antibiotics, surgery, and, more recently, the use of hyperbaric oxygen therapy where surgery is not always emergently needed with a high likelihood of success without any considerable adverse effects [10].
Case presentation
This case report is written in accordance with the guidelines for writing a clinical case report and principles of reporting case studies [11, 12]. A 35-year-old male patient presented to the emergency department of Jugole General Hospital, Harar, eastern Ethiopia. The patient presented with a history of crampy abdominal pain and distension for 2 days. It was accompanied by vomiting of ingested matter of four episodes per day. The patient had a failure to pass both feces and flatus for a daylong duration; and a history of cigarette smoking of 6 pack years, but no history of cough, fever, weight loss, or easy fatigability. The physical examination revealed blood pressure (BP) = 112/78, pulse rate (PR) = 104, respiratory rate (RR) = 25, SaO2 = 78% at atmospheric Air, 93% on face mask, body temperature (T0) = 36.9°C. He had dry lip and buccal mucosa, decreased air entry on right lower lung fields, grossly distended abdomen, hyperactive, hyper-tympanic percussion note, no tenderness, and empty rectum on digital rectal examination. Laboratory investigation showed an elevated white blood cell count of 19 750 with a left shift of 80%. In addition, an abdominal X-ray showed dilated small bowel loops with multiple air-fluid levels (Fig.1).
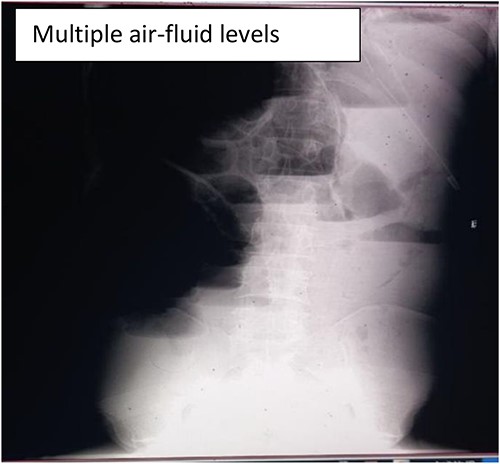
Abdominal X-ray showing dilated small with significant multiple air-fluid levels.
The patient was managed with a nasogastric tube for decompression, fluid replacement intravenously with normal saline of 0.9%, and ceftriaxone and metronidazole antibiotics as preoperative preparation. The patient was admitted for 5 days, and the abdomen was opened through a midline incision. There was about 400 ml of reactive intra-peritoneal fluid; 360° anti-clockwise rotated ilium over its mesentery and caused a closed loop obstruction of the ilium (Fig. 2). The whole small bowel was significantly dilated and multiple gas-filled cysts in the sub-serosal layer involving the proximal part of the bowel (distal jejunum and ileum 40 cm from the ileocecal valve) (Fig. 3). The dilated bowel pushed the diaphragm upward and the liver posteriorly and downwards, and viable bowel sandwiched between liver and diaphragm. The whole colon collapsed, and multiple mesenteric lymph nodes were noted, but there was no bowel ischemia. Reactive fluid was sucked out, the twisted small bowel was derotated, and the biopsy was taken from the cystic mass and mesenteric lymph nodes. The abdomen is closed in layers. The patient tolerated the procedure well. On face mask oxygen (15 L/min) until the 4th day, bowel function resumed on the third postoperative day, and diet (tea and porridge) was initiated and tolerated. Eventually, the patient was discharged on the 5th postoperative day from the hospital, and seen at the follow-up clinic on the 15th day after surgery, and was found to be well recovered. Biopsy result shows multiloculated cystic spaces filled with air which is compatible with pneumocystis cystoids intestinalis (Fig. 4).
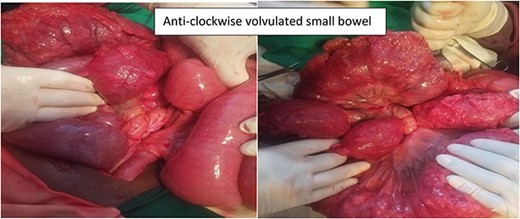
Intra-operative finding shows anticlockwise volvulated small bowel.
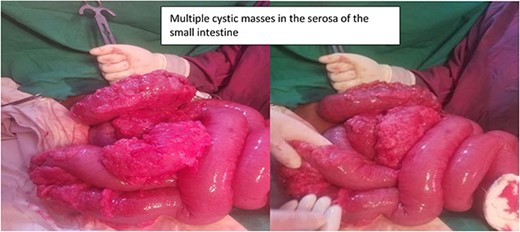
Intra-operative finding shows multiple air-filled cysts in the small bowel.
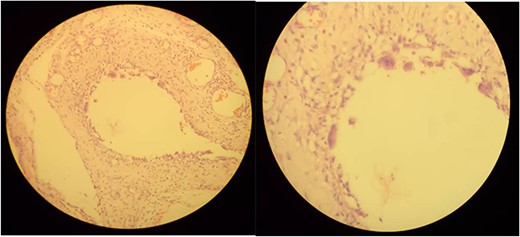
Microscopic examination shows variable-sized cystic spaces lined by thin fibrous stroma with focal multinucleated macrophages surrounding the space and intervening fibrous stroma with congested blood vessels and neutrophil mixed inflammatory cells.
Discussion
The case was initially treated as intestinal obstruction, and PCI was entertained intraoperatively, and confirmed with biopsy postoperatively. PCI may be associated with a range of gastrointestinal conditions, such as intestinal ischemia due to inflammatory bowel disease, endoscopic procedures, mechanical ventilation, and pulmonary diseases [13]. Approximately 85% of cases are thought to be secondary to coexisting disorders either in the gastrointestinal tract or the respiratory system [3, 6]. PCI can be secondary to inflammatory bowel disease, intestinal stenosis, drug use, extra-gastrointestinal diseases, abdominal trauma [14], scleroderma [15], and chronic obstructive pulmonary disease [6, 14, 16]. Management of the PCI was guided by the severity of the clinical presentation, which can vary based on the underlying cause [13]. Most consider its pathogenesis interrelated to a mechanical cause, like our case, due to intestinal obstruction explains the likely etiology where there is an increase in intra-luminal pressure and the accumulation of gas produced by aerogenic bacteria [7]. The presence of intestinal obstruction due to gastrointestinal tumors or any other condition that increases intraluminal pressure can promote the formation of cysts [8].
Patients with atypical symptoms and imaging manifestations tend to be misdiagnosed usually [9, 17, 18]. Because the differential diagnosis of PCI can be potentially life-threatening diseases like perforated viscus or necrotizing enterocolitis, one has to rule out the presence of such life-threatening surgical conditions as they might present with pneumo-peritonium (air in the peritoneal cavity) or pneumatosis intestinalis (air in the bowel wall). In contrast, misdiagnosis of PCI is common and can lead to unnecessary treatments and surgical procedures [5]. Given atypical clinical manifestations, it can be misdiagnosed as other diseases, such as polyps, inflammatory bowel disease, and even cancer, and biopsy was the confirmation test used [19], and it was gas-filled [20]. Cases with PCI are rarely suspected preoperatively and diagnosed intraoperatively, particularly in patients with intestinal obstruction [17]. For patients with primary PCI, a conservative approach with hyperbaric oxygen and antibiotics is the treatment of choice, and surgery is mandatory only for complicated diseases [2, 8]. This present case management was consistent with existing approaches such as intestinal ‘rest’, parenteral nutrition, antibiotics, fluids and electrolyte supplementation, and inhaled oxygen. Surgical intervention should be performed only in cases of bowel perforation, ischemia, or necrosis [3]. Although PCI patients have a high probability of death [3], the present has been discharged in good condition and recovered well during the 15th day of medical review.
Conclusion
PCI is a rare surgical emergency and resembles other life-threatening top surgical emergencies in a low-resource setting. Thus, conditions affect clinicians’ decisions on the identification of the case and treatment plan. So, clinicians should consider the possibility of PCI during investigation. PCI can be treated with hyperbaric Oxygen, but other life-threatening conditions should be ruled out in the context of low resource setting. Surgeons should be aware of the possibility of this condition as a result of bowel obstruction and have to avoid the costs and morbidities associated with unnecessary bowel resection or surgery.
Acknowledgements
We are so grateful to the healthcare workers at Jugole General Hospital and Hiwot Fana Comprehensive Specialized University Hospital, Haramaya University for the overall support. We would like to thank the patient for his willingness to take pictures while providing life-saving care.
Conflict of interest statement
The authors declare that they have no competing interest.
Funding
This report has not received financial support.
Author contributions
A.B., B.B., and M.A. contributed to the conception, acquisition of data, and interpretation of data and agree to be responsible for all aspects of the work. A.A. and H.U. were actively involved in for investigation and interpretation of the case. B.B., M.A., and A.S. drafted the manuscript. All authors reviewed the manuscript and incorporated their intellectual comments. All authors have read and approved the final manuscript.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Since this is a part of routine healthcare, ethical review is not applicable. Informed verbal and written consent were taken from the patient before surgery. The patient was informed about the pictures to be taken during surgery for teaching and research purposes, and the patient profile was de-identified.
Consent for publication
Written, signed, and informed consent was obtained from the patient for publication of the case report and accompanying images. A copy of the written consent is available and can be submitted upon reasonable request (MA: mezgebu.ab4@gmail.com).



