-
PDF
- Split View
-
Views
-
Cite
Cite
Jian-Rong Li, Da-Long Yang, Jin-Ming Wang, Wei Tian, Wei Wei, Cheng-Piao Luo, Lu-Nan Qi, Liang Ma, Jian-Hong Zhong, Importance of optimizing duration of adjuvant immune checkpoint inhibitor therapy to treat postoperative hepatocellular carcinoma after conversion therapy: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 11, November 2023, rjad610, https://doi.org/10.1093/jscr/rjad610
Close - Share Icon Share
Abstract
Patients with hepatocellular carcinoma at high risk of recurrence after hepatic resection or local ablation often undergo adjuvant immunotherapy with immune checkpoint inhibitors for 1 year in randomized controlled trials, but the appropriateness of this duration is controversial, especially given the risk of adverse events. Here we report the case of a 52-year-old Chinese man with initially unresectable multinodular recurrent hepatocellular carcinoma who underwent two cycles of transarterial chemoembolization, followed by hepatic resection and 24 months of adjuvant therapy with the PD-1 inhibitor tislelizumab. The patient achieved a recurrence-free survival time of 24 months, but he experienced elevated alpha fetoprotein, Grade 2 hypothyroidism and pruritus while on adjuvant therapy. This case highlights the need to optimize the duration of adjuvant immunotherapy after curative treatment for hepatocellular carcinoma in order to minimize risk of not only recurrence but also adverse events.
Introduction
Approximately 50%–70% of patients with hepatocellular carcinoma (HCC) experience recurrence within 5 years after potentially curative hepatic resection or ablation [1, 2]. Therefore, many patients receive adjuvant therapy after these curative treatments in order to improve long-term survival [3], and patients judged to be at higher risk of recurrence should be offered adjuvant immune checkpoint inhibitor (ICI) therapy, based on the newest guidelines from the American Association for the Study of Liver Diseases [4]. Several studies have linked such therapy to significantly longer recurrence-free or overall survival [5–8].
The application of conversion therapy to patients with unresectable HCC is increasing, but whether adjuvant ICI therapy is appropriate for such patients remains unclear [9–11]. Some work has suggested that adjuvant therapy can improve survival of patients who underwent conversion therapy [12]. Also, unclear is the optimal duration of such therapy. Here we describe a Chinese man with initially unresectable HCC who underwent conversion therapy followed by adjuvant ICI therapy. The patient achieved a recurrence-free survival time of 24 months, but he experienced several adverse effects because of the adjuvant therapy. This case suggests that while adjuvant ICI therapy can be effective after conversion therapy, its duration needs to be optimized.
Case presentation
A 52-year-old Chinese man was admitted to our hospital after physical examination revealed hepatic occupation that had persisted for the previous month. The patient had been chronically infected with hepatitis B virus for more than 20 years, and he had been diagnosed with hypertension 12 months previously, which he kept under control with nifedipine. Physical examination revealed no other unusual findings. His Eastern Cooperative Oncology Group score was 0, Child-Pugh score was 5, indocyanine green retention was 4.8% at 15 min, and level of alpha fetoprotein in serum exceeded 2000 ng/ml, for which no dilution test was performed (Supplementary Table 1). Contrast-enhanced magnetic resonance imaging revealed two massive nodular tumors in the liver, one at the junction of the left inner and upper right anterior lobes, and the other in the lower right posterior lobe (Fig. 1a and b).
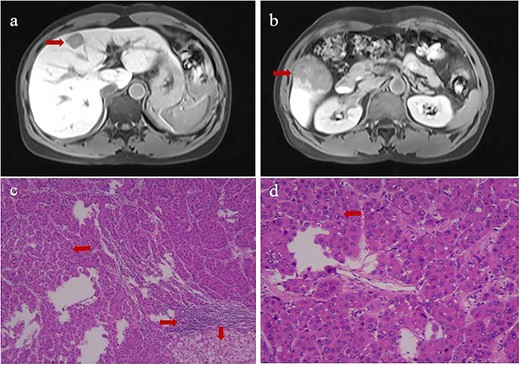
Contrast-enhanced magnetic resonance imaging at admission (upper row) and histopathology of primary tumors (lower row) of (a) the tumor at the junction of the left inner and upper right anterior lobes, and (b) the tumor in the lower right posterior lobe. Histopathology of the tumor in panels c and d showed tumor cells in Edmondson Grade II (leftward arrow), infiltration by macrophagocytes and lymphocytes (rightward arrow), and hepatocyte steatosis (downward arrows). Magnification, 40× in panel c or 100× in panel d.
The patient was diagnosed with HCC in Barcelona Clinic Liver Cancer Stage B. At 18 days after admission, the patient underwent hepatic resection of the right and middle lobes as well as cholecystectomy. Surgery proceeded uneventfully, and the patient recovered well. Postoperative histopathology confirmed that the two tumors were HCC and indicated an Edmondson grade of II (Fig. 1c and d). We detected one instance of microvascular invasion and one satellite nodule of Edmondson Grade II. Tumors expressed CK7 and glypican-3, but not CK19, based on immunohistochemistry. Surrounding liver tissue showed chronic changes associated with hepatitis, which we categorized as G1S1 on the inflammation grade (G) and fibrosis stage (S) [13].
We judged that our patient was at elevated risk of recurrence after resection because he had microvascular invasion, a satellite nodule, and multinodular tumors, so we administered one cycle of adjuvant transarterial embolization at 1 month after surgery in accordance with routine practices at our medical center [14, 15] and with Chinese HCC guidelines [16]. The patient was tested monthly for alpha-fetoprotein in serum, and he underwent contrast-enhanced computed tomography or magnetic resonance imaging every 3 months. He was also given antiviral therapy in accordance with Chinese guidelines [16]; we administered tenofovir because it may be more effective than entecavir [17]. The patient was discharged 1 week after surgery.
At 24 months after his admission with hepatic occupation, contrast-enhanced computed tomography detected intrahepatic recurrence, which was confirmed by magnetic resonance imaging. The patient was admitted again and given two cycles of transarterial chemoembolization (TACE), which achieved a partial response (PR) based on the Response Evaluation Criteria for Solid Tumors [18] (Fig. 2a and b). At 16 weeks after his second admission, the patient underwent right hemihepatectomy. Baseline variables before surgery are described in Supplementary Table 1. The surgery proceeded smoothly, and the patient recovered well. Postoperative histopathology revealed necrosis in more than 90% of the tumor, whereas no nodular cirrhosis, satellite nodules, or tumor emboli were observed. Instead, necrotic and proliferative fibrous tissue with multinucleated giant cells was observed, with abundant infiltration by lymphocytes and focal aggregation of neutrophils (Fig. 2c and d). Surrounding liver tissue showed chronic hepatitis changes (G2S2 on the inflammation and fibrosis scale) as well as hyperplasia of the small bile duct.
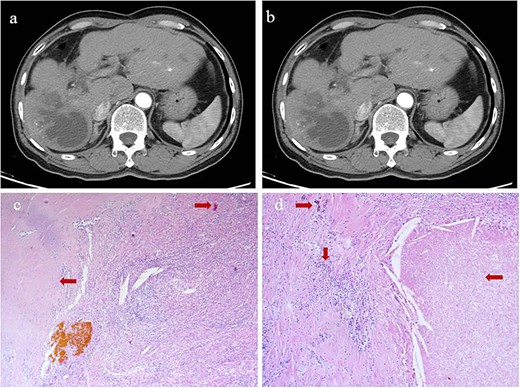
Intrahepatic recurrence and response to TACE. (a, b) Contrast-enhanced computed tomography in the (a) arterial phase or (b) venous phase after two cycles of TACE, showing PR of the recurrent tumor liver. (c, d) Histopathology of recurrent tumor after TACE at magnifications of (c) 40× or (d) 100×, showing necrosis of more than 90% of the tumor, proliferative fibrous tissue (leftward arrows), blue embolic agent in vasculature (rightward arrows), multinucleated giant cells, and abundant infiltration by lymphocytes (downward arrow).
Starting 1 month after repeat surgery, the patient received 200 mg of the ICI tislelizumab (BGB-A317; BeiGene, Beijing, China) once every 3 weeks via intravenous delivery lasting 60 min, in accordance with the manufacturer’s recommended dosing schedule. From 8 weeks on this therapy onwards, the level of alpha-fetoprotein in serum progressively increased. After 74 weeks on this therapy (21 treatments), the patient was diagnosed with Grade 2 hypothyroidism based on levels of thyroid hormone in serum and with Grade 2 pruritus based on signs and symptoms. Hypothyroidism was not treated because there were no obvious symptoms. However, he showed no signs of intra- or extrahepatic metastases in the entire body, abdomen, thoracic cavity, or brain based on magnetic resonance imaging or on contrast-enhanced, positron-emission, or bone-emission computed tomography. After 24 weeks on tislelizumab, a single metastasis was detected in the lung (Fig. 3). The patient was subjected to eight cycles of stereotactic body radiation therapy, and the level of alpha-fetoprotein in serum 3 weeks later was 275 ng/ml. Intensive follow-up continues.
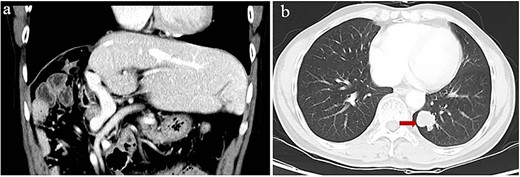
Computed tomography of lung metastasis after 2 years of adjuvant ICI. (a) Imaging at the venous stage did not reveal obvious lesions in the residual liver. (b) Imaging of the left lower lung revealed a single tumor (arrow).
Discussion
Here we present a patient with initially unresectable HCC who underwent hepatic resection after conversion therapy with two cycles of TACE, and then received 24 months of adjuvant therapy with the PD-1 inhibitor tislelizumab. The patient achieved a recurrence-free survival time of 24 months, but he experienced Grade 2 hypothyroidism and pruritus during adjuvant therapy. Immune-related adverse events are common in adjuvant immunotherapy [6, 19, 20]. Therefore, it is very important to optimize the duration of adjuvant ICI therapy that limits the occurrence of chronic immune-related adverse events without compromising survival outcomes.
There is no consensus on the duration of adjuvant ICI therapy after curative treatment for HCC, and current clinical trials tend to administer the therapy for 1 year [5, 21–23]. One year may be too long, because several studies suggest that the efficacy of ICIs does not last long: median progression-free survival time was only 2–8 months in various trials involving patients who received first-line ICIs on their own [24–26] or with tyrosine kinase inhibitors [27–30], or who received second-line ICIs with or without tyrosine kinase inhibitors [31–35] (Supplementary Table 2). Some non-randomized studies have administered adjuvant ICIs after hepatic resection for only 4–6 months, which was sufficient to achieve a significant reduction in risk of recurrence [6, 8].
Another reason to optimize the duration of adjuvant ICI therapy is that such inhibitors may increase risk of chronic adverse events related to the immune system [19, 20]. Monitoring and managing persistent toxic effects can be particularly cumbersome in HCC patients because such patients normally would not require close management because their cancer has been cured.
Our patient showed a recurrence-free survival time of 24 months after the first surgery (Fig. 4), slightly longer than the 20.3 months reported for patients receiving adjuvant hepatic artery infusion chemotherapy with 5-fluorouracil and oxaliplatin [36]. Our patient may also achieve recurrence-free survival of 24 months after repeat surgery: after nearly 24 months on adjuvant tislelizumab, no lesions were found on imaging even though the level of alpha-fetoprotein in serum remained slightly elevated. Such recurrence-free survive would be similar to the 25.2 months reported for patients at our medical center who received adjuvant ICIs with or without tyrosine kinase inhibitors after curative surgery [6].
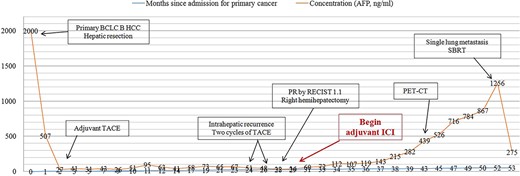
Concentration of alpha-fetoprotein in the patient’s serum over the course of clinical interventions. BCLC, Barcelona Clinic Liver Cancer; PET-CT, positron-emission tomography/computed tomography; RECIST, Response Evaluation Criteria in Solid Tumors; SBRT, stereotactic body radiation therapy.
The alpha-fetoprotein level in our patient’s serum began to rise rapidly after 8 months of adjuvant ICI treatment; after 16 months of treatment, lung metastasis was detected. Our experience highlights the usefulness of elevated alpha-fetoprotein as a marker of tumor recurrence, metastasis, or de novo growth [11]. Moreover, this case report highlights the need for non-inferiority clinical trials to explore shorter adjuvant ICI therapy to maximize benefit while minimizing risks. Lastly, our case justifies further studies into the effects of adjuvant ICIs on recurrence in HCC patients after curative treatment.
There were some limitations in this study. First, there was no needle biopsy for the recurrent tumor, and the tumor cells were almost necrotic after conversion therapy, so immunohistochemical analysis could not be performed. Therefore, significant tumor heterogeneity between the primary and the recurrent HCC cannot be ruled out because alpha-fetoprotein was not significantly elevated at the first HCC recurrence. We did not genetically profile the primary and recurrent tumors, which future work should do.
Patient perspective
The recurrence and metastasis of my HCC have burdened me and my family, and we thank my doctors for formulating a variety of effective treatments for me. We continued adjuvant immunotherapy beyond 1 year because the level of alpha-fetoprotein in my serum continued to rise, yet various types of imaging failed to detect any lesions. The prolonged immunotherapy led to immune-related adverse events. After consulting with my physician and reviewing the relevant medical literature myself, I see there is no consensus about the duration of effective adjuvant therapy.
Ethics statement
Ethical approval was provided for this study (LW2023130). The patient provided his written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author contributions
J.-R.L, D.-L.Y, J.-M.W, W.T, C.-P.L, L.-N.Q, B.-D.X, L.M, and J.-H.Z wrote the manuscript. All the authors were involved in medical care of the patient and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest statement
None declared.
Funding
The Specific Research Project of Guangxi for Research Bases and Talents (GuiKe AD22035057); the National Natural Science Foundation of China (82060510, 82260569); the Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor (Guangxi Medical University), Ministry of Education (GKE-ZZ202217, GKE-ZZ202311).
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Qin S, Chen M, et al. . IMbrave050 investigators. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial.



