-
PDF
- Split View
-
Views
-
Cite
Cite
Il D Kim, Eun J Ahn, Jung-won Yoon, Anna Choi, Sung H Joo, Retroperitoneal spindle cell tumor: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 10, October 2023, rjad601, https://doi.org/10.1093/jscr/rjad601
Close - Share Icon Share
Abstract
Spindle cell tumors exhibit a relatively low occurrence rate and can manifest in various locations within the human body, including soft tissues and bones. The process of making a diagnosis is supported by conducting pathological and immunohistochemical tests. A 50-year-old female patient visited the hospital with abdominal pain that lasted about a week. Magnetic resonance imaging of the pelvis showed that this mass was independent and was not a lymph node mass, but a retroperitoneal sarcoma type mass. As part of the treatment, the mass was surgically excised, and a supracervical hysterectomy was carried out. The tumor was wrapped in a grayish-white capsule and showed a lobulating pattern. Retroperitoneal spindle cell tumors, particularly those occurring in abdominal soft tissues, are infrequently observed. Histopathological diagnosis is done in stages, and when cases are ambiguous, immunohistochemistry can provide valuable guidance in the right direction.
Introduction
A spindle cell tumor is one of the various spindle-shaped cells that are round in the middle and pointed at the ends. Several types of tumors, including undifferentiated carcinoma, gastrointestinal stromal tumors, connective tissue tumors, and mediastinal tumors, can exist as spindle cells [1]. Spindle cell tumors have a low incidence and can occur in the soft tissues, bones, and other human body parts. Their morphological appearance may be carcinogenic or neoplastic [2]. Spindle cell tumors may be sarcomas or cancers.
Case presentation
A 50-year-old female patient visited our hospital with abdominal pain persisting for about a week. At present, the patient is in menopause, and there is no record of previous abdominal trauma, cancer, or any relevant family history. The levels of the β-subunit of human chorionic gonadotropin (1.19 mlU/ml), carbohydrate antigen 19-9 (21.5 U/ml), cancer antigen 125 (17.20 U/ml), and human epididymis protein 4 (54.9 pmol/L) were all within the normal range.
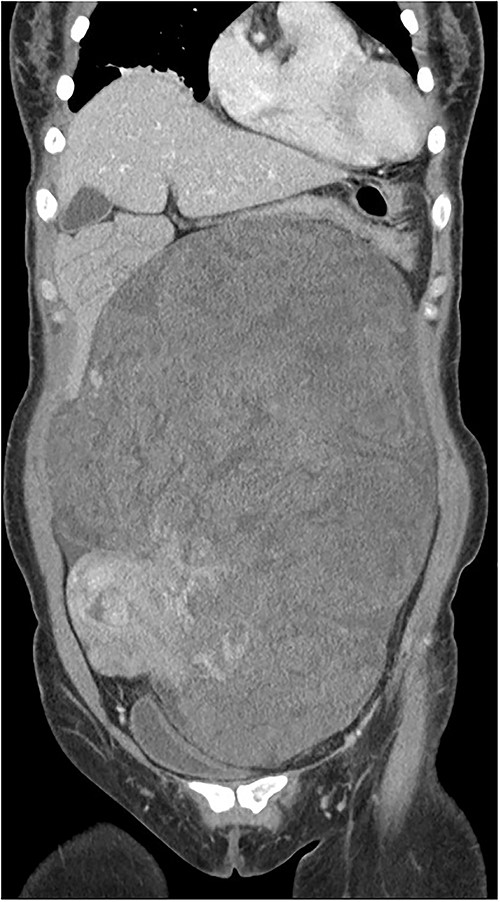
Computed tomography (CT) of the enhanced upper abdomen showed a well-defined, heterogeneous, focally enhanced soft tissue (30 × 20 × 9 cm) occupying the abdomen and pelvic cavity. The bowel and the uterus were displaced by the mass (Fig. 1). Magnetic resonance imaging of the pelvis showed that this mass was independent and not a lymph node mass but a type of retroperitoneal sarcoma. Positron-emission tomography–CT showed that a huge mass (32 × 23 × 12 cm) in the abdominopelvic cavity, with mostly heterogeneous mild 18F-FDG uptake.
During the surgical procedure, the general surgery team discovered a sizable, multilobulated, and hypervascular mass situated in the retroperitoneum. It occupied almost all parts of the abdominal cavity and was nearly in contact with the pelvic floor at the transverse colon level. The descending and sigmoid colon, rectum, uterus, and both ovaries were pushed to the right and attached firmly to the rear of the uterus. The mass was dissected gently from left lateral side of retroperitoneum to descending and sigmoid colon. Despite big size and thin wall, it was easily detached from colon and retroperitoneum. But it was found that the mass was firmly attached to rear of the uterus during dissection within pelvic cavity. The mass could not be removed with preserving the uterus (Fig. 2).
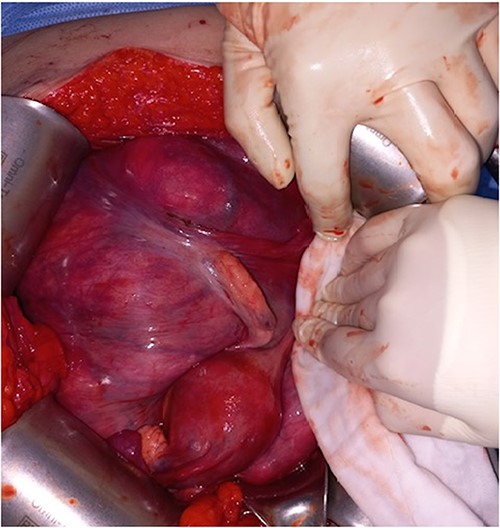
The descending and sigmoid colon, rectum, uterus, and both ovaries were pushed to the right and attached firmly to the rear of the uterus.
After being informed about the operation method by the medical staff, the patient opted for a supracervical hysterectomy. Regarding the obstetric and gynecological surgery findings, the uterus and adnexa were normal, and a supracervical hysterectomy was performed. The mass was successfully resected without any tumor rupture and without causing damage to adjacent organs. Finally, the operation was successful, lasting for 5 hours, with a total intraoperative bleeding of 200 ml. The patient's postoperative recovery was favorable, and there were no signs of recurrence or metastasis noted during the 12-month follow-up period.
The tumor was wrapped in a grayish-white capsule in a lobulated pattern, accompanied by bleeding; necrosis was not apparent. Yellow dot-like cellular components were observed. The findings for the spindle cell neoplasm (46.5 × 25.0 × 8.0 cm) were a smooth-muscle tumor of uncertain malignant potential (stump), with necrosis absent, a mitotic count <4/10 HPF, and moderate-to-severe and focal nuclear atypia (Fig. 3). Based on these results, the diagnosis revealed that the tumor originated from smooth muscle cells and was classified as a spindle cell tumor. Immunohistological analysis showed negative results for CD34, c-kit, PAN-CK, EMA, and S-100 and positive results for Desmin and SMA. The Ki-67 index was 5% (Fig. 4).
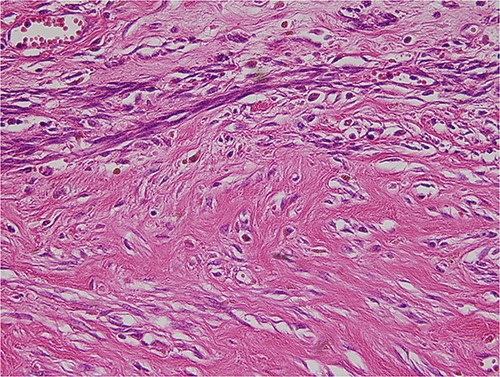
The tumor was composed of spindle cells with moderate to severe, focal nuclear atypia.
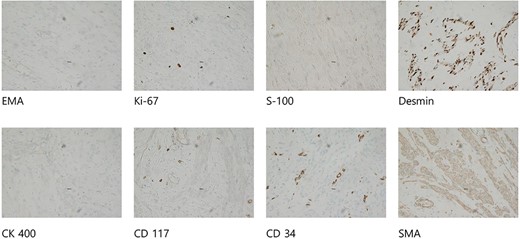
Immunohistological analysis showed negative results for CD34, c-kit, PAN-CK, EMA, and S-100 and positive results for Desmin and SMA. The Ki-67 index was 5%.
Discussion
Spindle cell tumors, which were first documented by Weiss and Enzinger in 1896, encompass a range of tumor types such as gastrointestinal stromal tumors, intra-abdominal aggressive fibromatosis, and fibrosarcoma [1]. In 1994, the World Health Organization formally categorized spindle cell tumors as soft tissue tumors [3]. Spindle cell tumors are also associated with various pathologies. Because of their rarity, no appropriate study has been conducted on their clinicopathological characteristics and diagnostic systems. Soft tissue tumors typically manifest in the extremities, while abdominal soft tissue tumors, particularly retroperitoneal fusiform tumors, are relatively rare occurrences [4]. Most of these occurrences happen at a young age, typically between 20 and 40 years, with no significant difference in frequency between men and women [5]. This mass typically develops in the extensive hidden area of the retroperitoneum and can grow to a considerable size without causing noticeable symptoms, leading to delayed diagnosis [6].
The primary approach for treating smooth muscle uterine leiomyomas and intra-abdominal spindle cell tumors is through comprehensive surgical intervention. Extensive radical resection can be performed if the tumor infiltrates neighboring organs within the abdominal cavity. The tumor was reported to exhibit biologically inactive behavior, with no evidence of remote metastasis, indicating a positive prognosis [1].
During evaluations, clinical examination, radiological diagnosis, and histologic findings often lack specificity and fail to provide conclusive information; therefore, pathological and immunohistochemical tests are important for a final diagnosis.
Differential diagnosis can be facilitated by employing immunohistochemical staining techniques with CD34 and C-Kit antibodies [7]. The use of the Ki-67 index is very important for identifying tumor cells with the morphological characteristics of spindle cells, smooth muscle cells, and fibroblasts [8]. In this patient, immunohistochemical staining for desmin and smooth muscle-specific actin was consistently positive; staining for CD34 and c-kit (CD117) was always negative, similar to the staining patterns of smooth muscle uterine leiomyoma. Differential diagnoses include other spindle-cell neoplasms, such as smooth muscle uterine leiomyomas, inflammatory myofibroblast tumors, vascular lipomas, and gastrointestinal stromal tumors.
A spindle cell tumor is considered malignant when the histopathological examination shows high cytoplasm, high mitotic activity (a mitotic count >4/10 HPF), polymorphism, necrosis, and hemorrhagic changes [9].
Conclusions
Retroperitoneal spindle cell tumors, particularly those located in abdominal soft tissues, are rare occurrences. Good results can be achieved through surgery following discussions in a multidisciplinary oncology committee and strict compliance with follow-up protocols. In histopathological diagnosis, a phased approach is used, and in ambiguous cases, immunohistochemistry can provide guidance in the right direction.
Conflict of interest statement
None declared.
Funding
No funding was required.
Data availability
All data (of the patient) generated during this study are included in this published article and its supplementary information files.



