-
PDF
- Split View
-
Views
-
Cite
Cite
Evan Gorgas, Shawn Dowling, Bezoar-induced small bowel obstruction: a rare cause of a common problem, Journal of Surgical Case Reports, Volume 2023, Issue 10, October 2023, rjad553, https://doi.org/10.1093/jscr/rjad553
Close - Share Icon Share
Abstract
Small Bowel Obstruction (SBO) is one of the most common diagnoses that general surgeons encounter. Adhesive disease, hernia, and neoplasm are the most common causes. A more rare cause is bezoar. A 66-year-old female with a history of prior abdominal surgery presented with clinical concern for SBO. CT scan of the abdomen and pelvis demonstrated SBO with a transition point in the left lower abdomen. The patient failed nonoperative management and was taken to the operating room for exploration. On exploration, a segment of hemorrhagic jejunum was found with an intraluminal bezoar. SBO secondary to bezoar can be managed endoscopically or operatively depending on location and size of the stone. Operative intervention can vary between laparoscopic milking of the bezoar distally, enterotomy with stone extraction, or bowel resection and anastomosis. This case illustrates the importance of maintaining a broad differential for common surgical disease processes.
Introduction
Small Bowel Obstruction (SBO) remains one of the most common diagnoses general surgeons encounter. The majority are caused by adhesive disease, hernia, neoplasm, and intussusception, however a small number are caused by bezoar. SBO secondary to bezoar has been reported at a frequency ranging between 0.4 and 4% [1]. Bezoars are insoluble intraluminal masses of undigested material that often originate in the stomach and pass into the small bowel via the pylorus [2]. The formation of stones often occurs in those who have inhibited gastric motility [3]. Medical causes include gastroparesis, stricturing Crohn’s Disease, hypothyroidism, and myotonic dystrophy [3, 4]. Surgical causes are prior antrectomy, pyloroplasty, vagotomy, or gastrojejunostomy [4–6]. SBO secondary to bezoar is difficult to diagnose at the onset as clinical presentation is not specific. Here, we present a case of an unexpected finding of bezoar-induced SBO in a patient with multiple prior abdominal surgeries.
Case report
The patient is a 66-year-old female with a history of laparoscopic abdominal surgery including appendectomy and cholecystectomy. The patient also reported two previous diagnostic laparoscopies but could not recall the reason the operations were performed. She presented to the emergency department with 2 days of worsening abdominal pain, nausea, belching, and distension. At presentation, she had not passed flatus or had a bowel movement in nearly 12 hours.
She was well nourished and did not appear to be in significant pain or distress. Her abdomen was soft, mildly distended with tympany, and was diffusely tender without rebound or guarding. She was hemodynamically normal. Significant laboratory values included a white blood cell count of 19 K/mcL, hemoglobin 14 g/dL, liver and renal function was within normal limits, potassium was 3.1 mmol/L, and lactate 2.0. She incidentally was found to have a urinary tract infection (UTI) on urinalysis. A CT scan with IV contrast demonstrated multiple dilated loops of small bowel with air fluid levels and a transition point in the left lower abdomen. There was no evidence of fecalization of the small bowel (Fig. 1). Given her hemodynamic stability and reassuring abdominal exam, the patient was admitted for nonoperative management of her SBO. She was also started on antibiotics for her UTI and plans were made for a Gastrograffin challenge the following day.
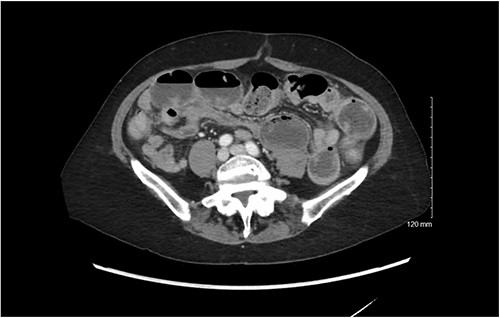
CT scan demonstrating dilated loops of small bowel with air fluid levels with distal decompressed loops.
On hospital day 1, the patient failed her Gastrograffin challenge due to emesis. During the same day, she also became hypotensive with a systolic blood pressure approximately 80 mmHg and required fluid bolus. Her blood pressure improved with the bolus but the decision was made to take the patient to the operating room for exploration. The plan was to perform diagnostic laparoscopy with possible conversion to open laparotomy. During laparoscopic exploration, no significant adhesive disease was noted. While running the bowel, ~30 cm distal to the Ligament of Treitz, a segment of grossly hemorrhagic small bowel with an intraluminal mass encountered. The mass was unable to be milked proximally or distally and the decision was made to convert to an open exploration. During exploration, the segment of hemorrhagic bowel (~20 cm) was resected and re-anastomosed in anatomic side-to-side functional end-to-end fashion. The rest of the abdomen was explored without any significant findings. The specimen was opened on the back table prior to closing the abdomen, which revealed a bezoar. Given concern for an underlying undiagnosed motility disorder, an EGD was performed, which demonstrated no notable findings.
Pathologic evaluation of the specimen ultimately showed a segment of jejunum with mucosal ischemic necrosis and ulcer, accompanied by a bezoar. The patient’s postoperative course proceeded uneventfully. On discharge, she was referred to GI for further outpatient work-up of motility disorders.
Discussion
Bezoars are classically divided into four subtypes that include phytobezoars (vegetable matter), trichobezoars (hair), pharmacobezoars (medication), and lactobezoars (milk proteins) [6]. As previously mentioned, reduced gastric motility and alterations in anatomy allow larger volumes of undigested food products to pass into the small bowel and become impacted downstream [4–6]. With modern imaging technology, CT is the primary modality used to diagnose this disease process. In a retrospective study of 72 patients with bezoar-induced SBO, Gao et al. found that bezoar shape, intestinal wall thickness, presence of mesenteric haziness, and mesenteric/peritoneal fluid as risk factors for failure of nonoperative management [7].
Treatment strategy is largely based on size, location, and chemical synthesis [6]. Chemical dissolution has been shown to be quite successful in the management of phytobezoars. Solvents such as saline solutions, hydrochloric acid, and Coca-ColaⓇ have been well described in the literature as effective dissolving agents [6]. Endo et al. describe the combination of dissolution with Coca-Cola Zero and endoscopic manipulation for the management of multiple bezoars in an elderly gentleman [8].
Proximal bezoars are almost universally best managed endoscopically [6]. When still in the stomach, bezoars can undergo mechanical fragmentation making extraction easier. Similar techniques have been described in those with rectal bezoars as well [9]. When there is concern for small bowel bezoar, surgery remains the best treatment modality. In a series of 40 patients treated for bezoar-induced SBO, Wang et al. demonstrated that those with large volume bezoars (5.3 cm length and 3.7 cm length) and those with higher density bezoars on CT scan were independent risk factors for requiring surgical intervention [2]. When surgery is needed, the most common operations performed are partial small bowel resection if there is concern for bowel compromise, enterotomy with bezoar extraction, or milking the stone toward the ileocecal valve [2, 10]. Furthermore, it is possible to fragment the stone while it remains intraluminal and milk the fragments forward [6]. Though milking the stone toward the valve eliminates the need for resection and anastomosis, it does carry the risk of intraluminal bleeding, scarring, and there is a small risk it may not pass [2].
Conclusion
This case illustrates a rare cause of SBO in a patient that would be expected to have obstruction secondary to adhesive disease from prior abdominal surgeries. When treating bezoar, it is important to consider all available modalities.
Conflict of interest statement
There are no conflict of interests to declare.
Funding
No funding was utilized in the preparation of this manuscript.
Patient consent
Written and verbal consent were obtained from the patient for presentation of this case. Written consent can be provided to the Editor upon request.



