-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher J Salgado, Daisy I Gonzalez, Desha Gelles-Soto, Adan Mercado, Use of allograft fat for aesthetic and functional restoration of soft tissue contour deformities, Journal of Surgical Case Reports, Volume 2023, Issue 1, January 2023, rjac629, https://doi.org/10.1093/jscr/rjac629
Close - Share Icon Share
Abstract
The authors report a case series of five patients with Leneva grafted into the nose, hand, genitalia and below-the-knee stump. Leneva is an allograft adipose matrix derived from aseptically processed human adipose tissue with retained matrix proteins, growth factors, cytokines and collagens. It is manufactured hydrated and is available in pre-loaded syringes. Five patients (3F, 2 M) with a mean age of 50.7 (range 31–77 years) injected with a mean volume of 4.2 cc (range 3–6 cc) of Leneva in various anatomic locations with an average follow up time of 4.25 months (range 0.5–12 months) experienced no allergic reactions, infection, fat necrosis or oil cysts. All patients were pleased with the restoration of fullness to the injected site. The authors believe that Leneva is a promising multi-use filler for restoring soft tissue defects following resection of tumours, to rejuvenate age-related atrophy, aesthetically enhance the genitals and provide padding for transtibial prostheses.
INTRODUCTION
Leneva (Musculoskeletal Tissue Foundation, Branch, NJ), previously marketed as Renuva, is a revolutionary allograft adipose matrix (AAM) derived from aseptically processed human adipose tissue stripped of immunogenic lipid and cellular components. It is a safe, off-the-shelf solution with clinical applications to restore age related volume loss in the face, body and hands, and can also be used to treat diabetic foot ulcers, pressure ulcers, tunnelling wounds and fat pad atrophy. Endogenous matrix proteins, growth factors (adiponectin, leptin, angiopoietin, insulin growth factor-1 and fibroblast growth factor-1 and -2), cytokines and collagens I–VI are adipogenic and angiogenic factors naturally found in adipose tissue and preserved in Leneva to support the adipocyte regeneration process [1]. These factors support neovascularization, stem cell differentiation to adipocytes, and modulation of glucose and lipid metabolism [2]. When injected, the natural factors that are retained in the matrix support host cell infiltration and revascularization of the matrix, making Leneva an ideal substance to fill in soft tissue defects due to its ability to integrate with surrounding tissue, maintain the viability of the implanted fat and structurally accommodate adipogenesis.
Leneva is manufactured hydrated and is available in pre-loaded syringes. After 10 passes into an empty syringe through a Luer-Lok connector, it can be injected into the subcutaneous space anywhere in the body, where it will promote the organization of infiltrating endothelial cells, stimulate angiogenesis and act as a scaffold to support adipocyte differentiation and growth [3]. The advantages of Leneva over autologous fat grafting following liposuction includes decreased donor site morbidity, shorter operating time, decreased anaesthesia and cost—as insurance commonly does not cover liposuction. Furthermore, the results from autologous fat grafting are dependent on surgical technique and can be complicated by fat necrosis, infection, fibrosis, low retention and oil cysts [4]. The purpose of this article is to describe the use of Leneva as a soft tissue filler to restore the soft tissues following resection of tumours, rejuvenate age-related atrophy, aesthetically enhance the genitals and provide padding for transtibial prostheses.
MATERIALS AND METHODS
Five consecutive patients in whom Leneva AAM was used between April 2021 and March 2022 have been included in this study under the care of a single surgeon. Table 1 illustrates indications and anatomic locations of Leneva AAM injections. Enrollment in the study was limited to subjects who met the following inclusion criteria: males or non-pregnant non-breastfeeding females, age 22 or older. Subjects were not permitted to be enrolled in the study if they met any of the following exclusion criteria: history of allergy to lidocaine or other amide-type anaesthetics.
| Anatomic locations . | Indications . | Total amount of Leneva injected . |
|---|---|---|
| Nose | Cancer resection | 3 cc |
| Labia Majora | Atrophy | 3 cc |
| Dorsum of hands | Atrophy | 3 cc |
| Penile shaft | Atrophy | 6 cc |
| Left knee stump | Padding | 6 cc |
| Anatomic locations . | Indications . | Total amount of Leneva injected . |
|---|---|---|
| Nose | Cancer resection | 3 cc |
| Labia Majora | Atrophy | 3 cc |
| Dorsum of hands | Atrophy | 3 cc |
| Penile shaft | Atrophy | 6 cc |
| Left knee stump | Padding | 6 cc |
| Anatomic locations . | Indications . | Total amount of Leneva injected . |
|---|---|---|
| Nose | Cancer resection | 3 cc |
| Labia Majora | Atrophy | 3 cc |
| Dorsum of hands | Atrophy | 3 cc |
| Penile shaft | Atrophy | 6 cc |
| Left knee stump | Padding | 6 cc |
| Anatomic locations . | Indications . | Total amount of Leneva injected . |
|---|---|---|
| Nose | Cancer resection | 3 cc |
| Labia Majora | Atrophy | 3 cc |
| Dorsum of hands | Atrophy | 3 cc |
| Penile shaft | Atrophy | 6 cc |
| Left knee stump | Padding | 6 cc |
Adipose allograft implantation technique
Leneva comes with instructions and two sterile packages containing a syringe preloaded with 3 cc of AAM tissue and another containing three empty 3 cc syringes and a sterile Luer-Lok connector. Using sterile technique, 1.5 cc of AAM and the contents were passed back and forth for a minimum of 10 passes, as per instructions. This step was repeated using the remaining AAM and another empty syringe. Each patient received local anaesthesia in the region where they were to be injected with Leneva with a 20-gauge needle.
RESULTS
Five patients (3F, 2 M) with a mean age of 50.7 (range 31–77 years) injected with a mean volume of 4.2 cc (range 3–6 cc) of Leneva in various anatomic locations with an average follow up time of 4.25 months (range 0.5–12 months) experienced no allergic reactions, infectious complications, fat necrosis or oil cysts All patients were pleased with the restoration of fullness to the injected site and noted an improvement in the quality of their skin (Figs 1–10).
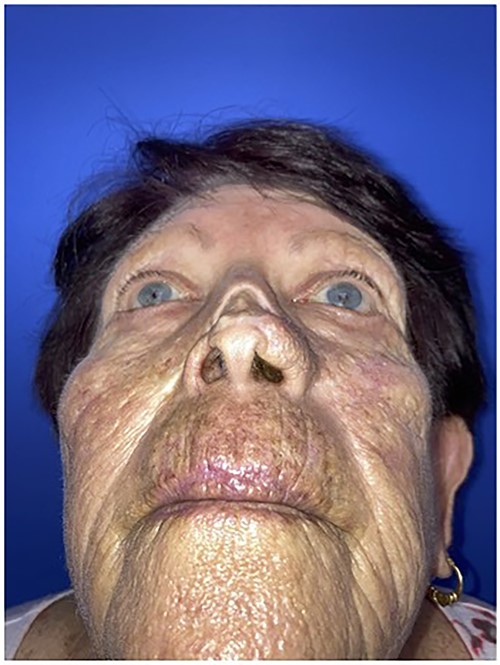
Worm’s eye view of the patient demonstrating nasal contour deformity due to squamous cell cancer resection and skin graft repair.
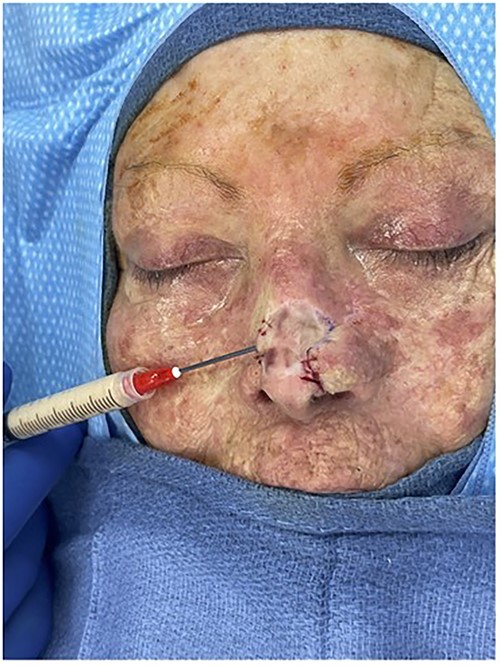
Intraoperative photo injecting Leneva AAM into the patient’s nasal defect.
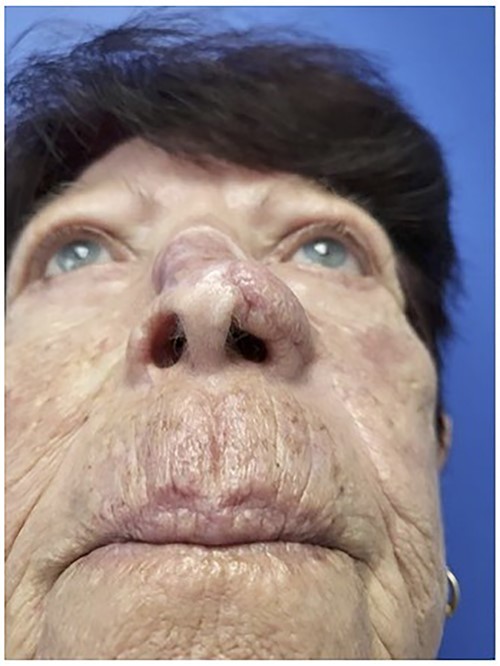
Worm’s eye view demonstrating incorporation of Leneva into the nasal defect.

Pre-operative view of the patient showing labia minora hypertrophy and labia majora atrophy.

Post-operative view of the patient following labia majora augmentation with Leneva.
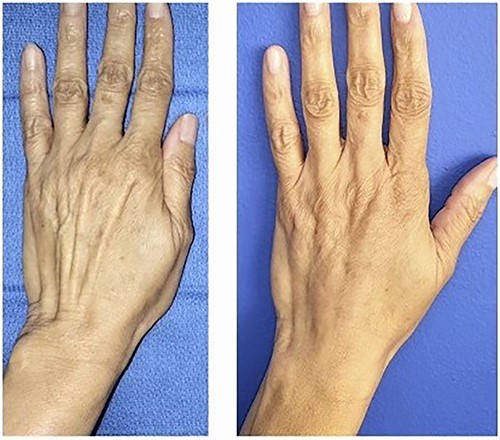
Before and after photo after injecting the dorsum of the left hand with Leneva.
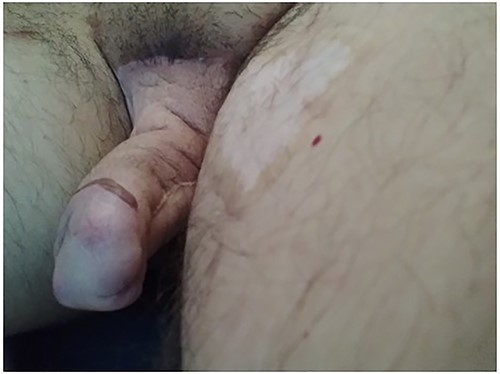
Pre-operative view of the patient’s neophallus with fat atrophy.
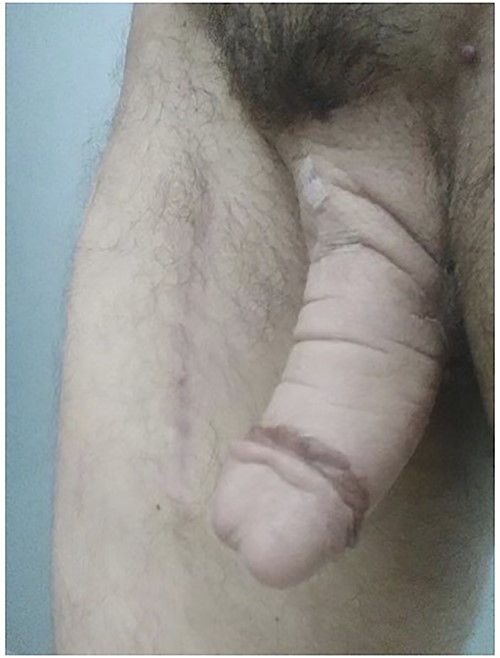
Post-operative view of the patient’s neophallus after Leneva injection.
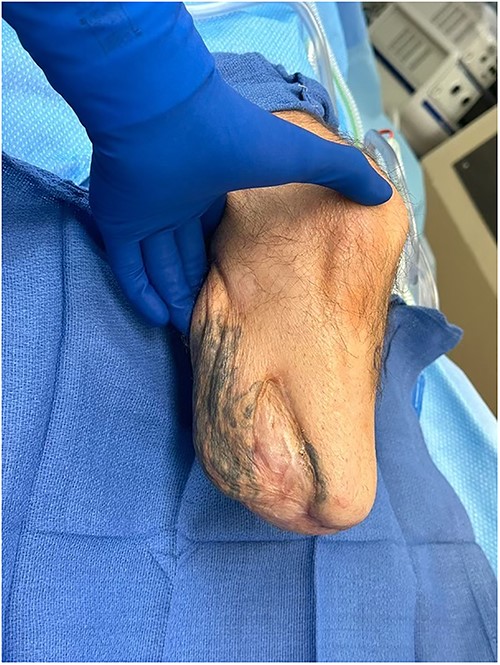
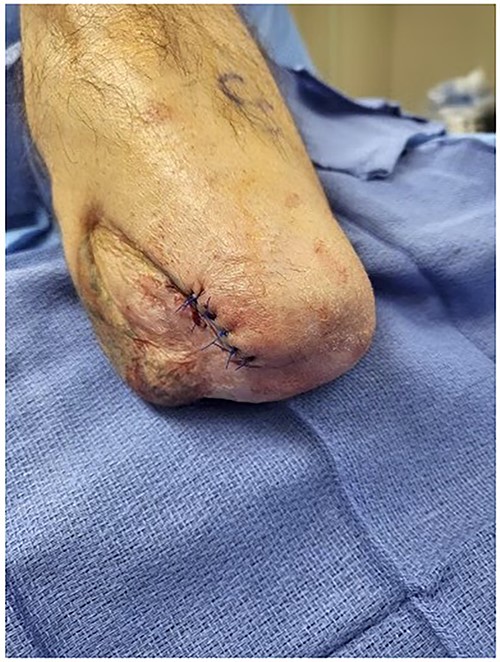
Post-operative view of the patient’s left below-the-knee stump after Leneva injection.
Case #1
The first case is a 77-year-old woman with a history of multiple squamous cell cancers (SCC) of the face. An SCC lesion twice removed from the dorsum of the nose left a large defect which was initially repaired with a split thickness skin graft at an outside institution. The patient presented with a contracted skin graft over cartilage deformity 1 cm × 1 cm × 1 cm directly over the dorsal cartilage in the middle third of the nose (Figs 1 and 2). She complained of significant pain and unsightly appearance. Following dissection of the graft off the cartilaginous structures, 3 cc of Leneva was injected deep to the skin graft above the nasal cartilages (Fig. 3). The skin graft was advanced cephalad and tissues at the left lateral nasal ala were advanced towards each other for total tissue rearrangement of 4 cm. The volume over the nasal dorsum clinically improved from baseline and was retained four months post-operatively (Figs 4 and 5). Skin tone, texture and overall appearance improved. The patient was satisfied with the results, and there were no adverse events.
Case #2
The second case is a 31-year-old nulliparous woman with no significant past medical history and BMI of 20, who was referred to Constructive Surgery Associates seeking vaginal rejuvenation. On pre-operative exam, the patient had labia minora hypertrophy and labia majora atrophy (Fig. 6). After excess labia minora and clitoral hood tissues were resected, 1.5 cc of AAM was injected into the left and right labia majora using a 20-gauge needle. Once infiltration was complete, homogeneous distribution of the filler with mild massage was performed. A total of 3 cc of Leneva was injected into the patient. With a single treatment, the volume of the labia majora clinically improved from baseline and was retained 12 months post-operatively (Fig. 7).
DISCUSSION
The results of this preliminary case series suggests that Leneva is not only a promising material for filling soft tissue facial defects following cancer resection, but also an exciting tool for genital/hand rejuvenation and to improve the comfort for patients with transtibial prostheses. To our knowledge, this is the first report demonstrating the use of Leneva on a nasal defect, on the genitals for aesthetic enhancement, and on a BKA stump. There is a paucity of literature on Leneva’s use in facial defects. In a study by Rohich et al., Leneva was shown to augment temple atrophy in middle aged healthy women and histologically demonstrated autologous fat formation, angiogenesis and tissue remodeling at 8, 12 and 16 weeks [5]. Although other injectable fillers such as hyaluronic acid provide similar effects as Leneva, offering temporary benefit for 3–12 months [6], the results from Leneva are anticipated to be much longer lasting, as adipocytes have an average lifespan of 10 years [7].
Kokai et al. have demonstrated the angiogenic and adipogenic properties of Leneva through in vitro cell-matrix studies [8], in vivo implantation in a preclinical murine model [8] and clinical evaluation [9]. Human adipose derived stem cells seeded on the AAM, attached, infiltrated and proliferated on the matrix, differentiating into adipocytes as evidenced by cell morphology, secretion of adipogenic matrix proteins, and lipid accumulation [8]. In immunocompromised and immunocompetent mouse models, Kokai et al. and Giatsidis et al. demonstrated the ability of AAM to form new adipose tissue [8, 10]. Moreover, Kokai et al. assessed the safety of AAM on the non-dominant wrists of human subjects, reconfirming histologic results of adipogenesis [8]. In her most recent evaluation, 10 women underwent grafting of 120 ml of AAM into the abdominal pannus with longitudinal biopsies. Within the 6-month time span of the study, it was found that in clinically relevant volumes, the material is not only safe and cell compatible, but also provides a cell scaffold that is reorganized over time with increasing density of newly generated perilipin-expressing adipocytes [9]. These results suggest that this off-the-shelf matrix contains components that permit cell attachment and migration as well as adipogenic instructive cues—making it a viable time- and cost-saving replacement for adipose tissue harvesting and lipografting.
Furthermore, Leneva has been shown to relieve pressure and pain from diabetic pressure ulcers and pedal fat pad atrophy [11, 12]. Preliminary experiences suggest that the reduction in tissue stress of pre- and post-ulcerative lesions may extend ulcer-free days for patients in diabetic foot remission.
CONCLUSION
Our report illustrates the clinical applications of Leneva AAM to provide ease of use in a low-cost setting to augment soft tissue defects for a wide array of cutaneous pathologies. Although our report is limited to five case studies lacking histological analyses, our clinical results indicate a novel application to skin cancer reconstruction, genital enhancement, hand rejuvenation and padding for BKA stumps. Leneva provided volume retention and was well tolerated by all five patients, with no adverse side effects. This encouraging result justifies larger studies to further evaluate the safety and efficacy of the AAM for the correction of soft tissue facial defects following cancer removal, soft tissue augmentation for the genitals and hands, and padding for transtibial prostheses.
STATEMENT OF FINANCIAL DISCLOSURE
The authors have no financial disclosures related to this research manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.



