-
PDF
- Split View
-
Views
-
Cite
Cite
Carolina Baz, Nicolas H Dreifuss, Antonio Cubisino, Francisco Schlottmann, Alberto Mangano, Gabriela Aguiluz, Carolina Vanetta, Mario A Masrur, Pier Cristoforo Giulianotti, May the robotic approach expand the indications for redo surgery in recurrent pNETs in Multiple Neuroendocrine Neoplasia type 1?, Journal of Surgical Case Reports, Volume 2022, Issue 9, September 2022, rjac433, https://doi.org/10.1093/jscr/rjac433
Close - Share Icon Share
Abstract
Pancreatic neuroendocrine tumors (pNETs) represent the leading cause of disease-specific mortality in patients with Multiple Neuroendocrine Neoplasia type 1 (MEN1). Although surgery is the recommended treatment for non-functional pNETs >2 cm, the management of recurrent lesions between 1 and 2 cm is controversial. Robotic surgery was used on a 29-year-old female with MEN1 and previous distal splenopancreatectomy that presented with a 1 cm recurrent pNET. The advantages offered by this approach facilitating a precise resection of the tumor and minimizing the postoperative morbidity may favor the decision towards redo surgery for local recurrences <2 cm, expanding current indications.
INTRODUCTION
Pancreatic neuroendocrine tumors (pNETs) affect up to 85% of patients with multiple endocrine neoplasia type 1 (MEN1) [1, 2]. Unlike sporadic cases, pNETs associated with this syndrome are usually multiple, nonfunctioning and related to a higher risk of recurrence and metastasis. Although surgical excision remains the standard therapy for localized lesions >2 cm, the treatment of recurrent lesions between 1 and 2 cm is controversial [2, 3]. Some believe that the high risks of complications associated with redo surgery outweigh the benefits [2, 4]. On the contrary, other groups recommend an early reoperation to achieve long-term disease-free survival [3, 5]. The robotic system may provide a solution allowing aggressive management while minimizing the complications related to reoperations [6, 7]. Still, scarce information is available regarding the ideal candidates for the surgical approach, the optimal timing and the type of procedure to be done.
CASE REPORT
The patient is a 29-year-old female with a history of MEN1, stage II (pT3N0) pancreatic tail neuroendocrine tumor, hyperparathyroidism and recurrent episodes of kidney stones. She underwent a robotic-assisted distal splenopancreatectomy and, after 18 months of follow-up, a magnetic resonance imaging showed a 1 cm lesion in the pancreatic body (Fig. 1A and B). An endoscopic ultrasound reported a 13 × 10 mm solid lesion (Fig. 2). A biopsy was taken, and the histopathology informed a neuroendocrine tumor recurrence.
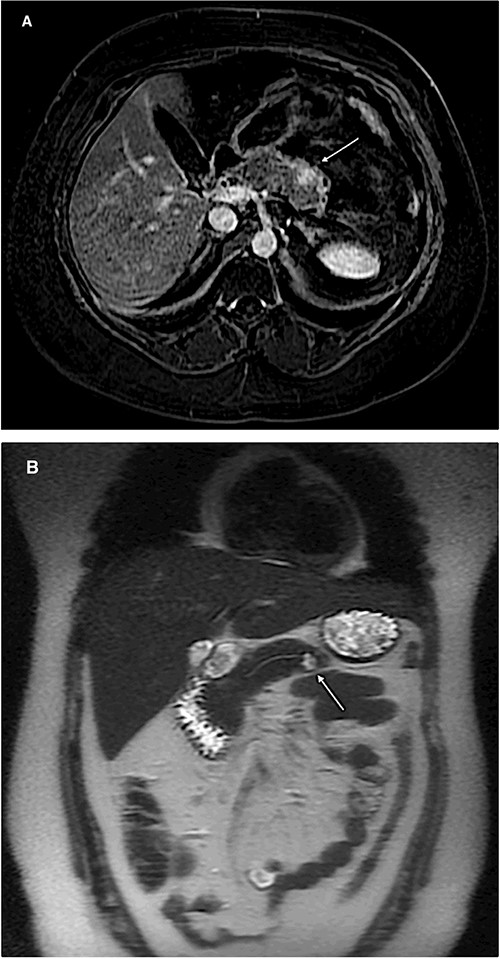
Magnetic resonance imaging of the abdomen (A: axial section, B: coronal section) showing a 1 cm lesion in the remnant pancreatic body.
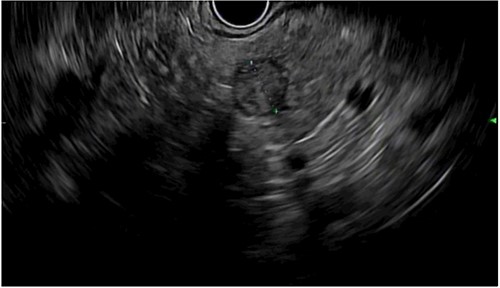
Endoscopic ultrasound showing a 13 × 10 mm solid lesion in the pancreatic remnant.
A robotic redo distal pancreatectomy was performed. Adhesions from the previous operation were taken down and the pancreatic stump was dissected. Samples of lymphatic tissue were sent for permanent pathology. An intraoperative ultrasound scan was done to localize the tumor and rule out concomitant lesions. A 2-cm nodule was found in the pancreatic stump (Fig. 3). With precise dissection, the gland was mobilized, reaching the confluence of the splenic and the portal vein. Afterward, the pancreatic stump was resected using a linear stapler (Fig. 4A and B). Once hemostasis was obtained, fibrin glue was applied to the section line and two drains were left close to the stump (Fig. 5). The patient was discharged on postoperative day 7. She developed a pancreatic biochemical leak that was managed conservatively. The histopathology analysis showed a grade 2, 1.3 cm well-differentiated neuroendocrine tumor with a low mitotic rate and Ki 67 index of 3%. After 16 months of follow-up, there was no evidence of recurrence.
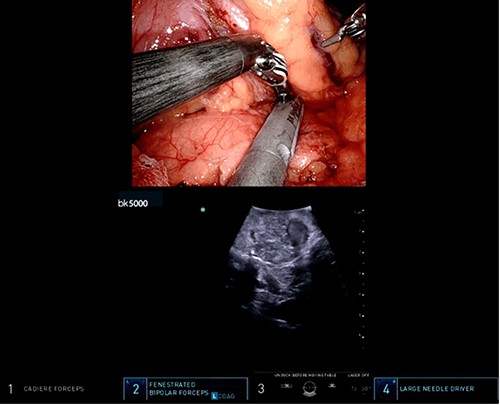
Intraoperative ultrasound revealing a 2 cm lesion in the pancreatic stump.
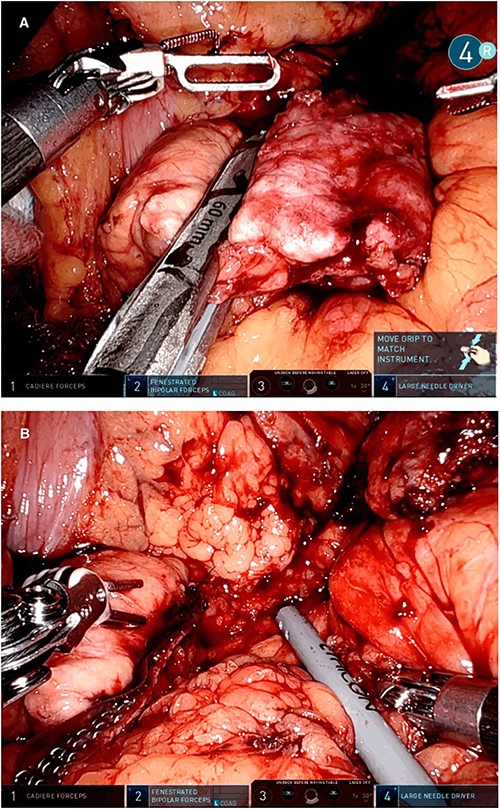
Intraoperative picture showing the pancreatic resection with a linear stapler (A) and the pancreatic stump with the stapler reinforcements in the resection line (B).
DISCUSSION
pNETs represent the leading cause of disease-specific mortality in patients with MEN1 [8]. For the past years, there has been an increasing interest in the behavior of these tumors since they are associated with malignant potential. Different predictors such as size, lymph node involvement and the presence of metastasis have been associated with poor survival [2, 8]. Surgery is considered the only curative treatment and is recommended for nonfunctioning pNETs >2 cm by the main guidelines [9, 10]. Lesions <1 cm are usually observed if an adequate surveillance program can be implemented. Lastly, for pNETs measuring 1--2 cm, the decision between observation and resection is individualized according to the patient’s and tumor’s characteristics (grade, Ki-67 index, growth rate) [4, 11].
When associated with an autosomal dominant endocrine tumor syndrome, pNETs have an earlier onset (30–50 years of age vs. 50–80 years), are usually nonfunctioning, multifocal, and recurrence affects up to 42% of the patients [1, 2]. In some studies, this percentage is even higher. For instance, Bartsch et al. [1] reported that 73% of MEN1 patients developed a new pNET in the pancreatic remnant after surgery. Distant metastasis represent the most frequent pattern of recurrence in pNETs and are associated with a decrease in the survival rate. Weber et al. [12] found that patients with liver metastasis had a significant decrease in survival (30 vs. 96 months) when compared with those without them. In addition, several studies [1, 3] have reported that early surgery seems to prevent their development.
For some authors, the elevated risks associated with reexploration exceed potential benefits [2, 4]. Others believe that surgical treatment should be attempted notwithstanding the higher chances of complications given the extended disease-free periods [1]. Currently, there are no specific recommendations regarding the indications for surgical reexploration or the type of reoperation. Fendrich et al. [3] considered newly developed pNETs or local and distant metastasis as indications for reoperations and they involved enucleations, distal pancreatic resections, duodenotomy, pylorus-preserving pancreaticoduodenectomy or resection of metastasis alone. As for the complications related to surgical reinterventions, a higher rate has indeed been reported. Some studies [1, 3] published that the morbidity increased from 36% after the initial operation to 57% for the third to fifth reoperation, being pancreatic fistula the most common complication. An increase in mortality has also been mentioned, being 2–3% after the first reoperation and reaching 29% after the third one. However, the minimally invasive approach may counter these drawbacks. The implementation of less invasive procedures has increased due to the benefits outlined in multicenter studies and recent randomized trials [6, 13]. The robotic platform incorporates the advantages of minimally invasive surgery regarding less intraoperative blood loss, hospital stay and morbidity while adding specific features such as a 3D magnified view and Endowrist instruments [14, 15]. Properties like high dexterity and enhanced vision may help to obtain better results not only when compared with the open approach [7] but even when analyzed against laparoscopic surgery since improved oncological outcomes, superior lymph node collection and higher spleen preservation have been reported [14]. Considering the favorable survival rates among these patients after redo-surgery, when feasible, a robotic-assisted reoperation should be considered. Fendrich et al. [3] and Norton et al. [5] both concluded that, in their experience, an early reoperation improves the duration and quality of life. The latter study reported an 80% 5-year survival, suggesting the benefits of redo procedures. Robotic surgery may be the key to the concerns related to a higher risk of complications that may have hampered the indication of early aggressive management in recurrent pNETs <2 cm.
Further investigation is needed as well as studies with long-term follow-up to provide a better understanding of these tumors’ behavior and patient’s outcomes. As a result, specific criteria may be developed to guide future decisions.



