-
PDF
- Split View
-
Views
-
Cite
Cite
Natalie Chen, K V Chalam, Successful management of a rare case of endogenous endophthalmitis from soft tissue (gluteal) abscess, Journal of Surgical Case Reports, Volume 2022, Issue 9, September 2022, rjac412, https://doi.org/10.1093/jscr/rjac412
Close - Share Icon Share
Abstract
Endophthalmitis, an ophthalmic emergency carries poor visual prognosis without prompt diagnosis and treatment. Endophthalmitis is often exogenous and rarely endogenous. In this report, we describe a rare case of bacterial endogenous endophthalmitis in an immunocompetent 51-year-old patient due to methicillin-resistant staphylococcus aureus septicemia from a gluteal abscess. Ultrasonography confirmed endogenous endophthalmitis. The patient was treated with immediate intravitreal antibiotic injections, prolonged intravenous antibiotics in association with pars plana vitrectomy, retinal detachment repair and vitreous debris removal. Successful treatment was confirmed with negative blood cultures and a clear vitreous on b-scan ultrasound and fundus photography with improvement of his visual acuity. This case highlights the importance of consideration of rare infectious foci as etiology and prompts treatment for successful resolution of endogenous endophthalmitis.
INTRODUCTION
Endophthalmitis, a rare ophthalmic emergency can rapidly progress to blindness without prompt diagnosis and aggressive treatment. It is endogenous or exogenous based on the route of infectious transmission. Exogenous endophthalmitis is far more prevalent (92–98%) and results from direct inoculation from intraocular surgery or trauma. Endogenous endophthalmitis (EE) is rare (2–8%) [1] and results from hematogenous spread of microorganisms [fungal (65.9%) or bacterial (34.1%)] from an extraocular site to the eye.
EE is much more likely to develop from fungemia (0.4%) than bacteremia (0.03%); Immunocompromised status, HIV, diabetes mellitus and malignancy are common predisposing conditions [2]. The most common source of infection is endocarditis, followed closely by urinary tract infections, indwelling catheters and infection from dialysis vascular access [3]. However, EE from soft tissue infections is rare [4].
In this report, we describe a unique case of bacterial EE in an immunocompetent patient from bilateral gluteal abscess that was successfully managed with prompt intravitreal antibiotics and pars plana vitrectomy. To our knowledge, this is the first reported case of EE from soft tissue (gluteal) abscess.
CASE SUMMARY
A 51-year-old Caucasian male presented to the emergency department with sudden loss of vision of 3-day duration in left eye, associated with severe eye pain and worsening bilateral gluteal abscesses with drainage. His medical history was significant for insulin-dependent type 2 diabetes mellitus, hyperlipidemia, intravenous (IV) drug use and recurrent gluteal abscess that was successfully treated 2 months earlier with systemic antibiotics. His ocular history was significant for dense corneal opacity (from healed corneal ulcer) with complete loss of vision in his right eye.
On examination, his best corrected visual acuity was light perception right eye (LP OD) and hand movements left eye (HM OS). Intra ocular pressure (IOPs) were 8 mm Hg OD and 10 mm Hg OS. Pupillary reflex was brisk without afferent pupillary defect left eye (APD OS). Slit lamp examination of his left eye revealed clear cornea, 3 mm layered hypopyon with 4+ cell in the anterior chamber and mild cataract. Fundus could not be visualized from dense exudation in OS and from diffuse corneal opacity OD; Ultrasonography (USG) demonstrated dense vitritis (Fig. 1A) and computed tomography scan with contrast revealed thickening and enhancement of the scleral margins of the left ocular globe consistent with endophthalmitis (Fig. 1B and C).
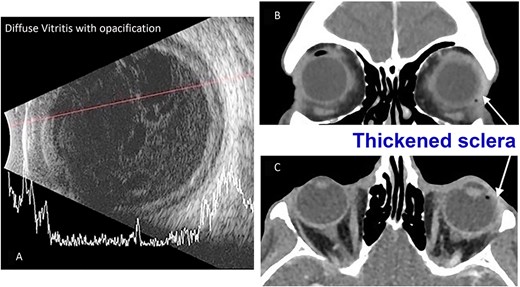
(A) B-scan USG of the patient’s left eye taken during their initial emergency department presentation demonstrating significant vitreous debris consistent with endophthalmitis. (B) Maxillofacial coronal postcontrast CT scan demonstrating left eye scleral thickening more prominent laterally (arrow) and globe asymmetry. (C) Axial postcontrast image of the orbits demonstrating left eye scleral thickening more prominent laterally (arrow) and globe asymmetry.
Systemic examination revealed left gluteal abscess (Fig. 2A) and right chronic gluteal wound (Fig. 2B). Incision and drainage of left gluteal abscess revealed two cavities of non-purulent fluid (4 cc drained). After obtaining blood cultures, patient was started on IV vancomycin, IV piperacillin-tazobactam. His hospital course was complicated by persistent positive blood cultures of methicillin-resistant staphylococcus aureus (MRSA), tricuspid valve infectious endocarditis, multiple septic pulmonary emboli and necessitated continued administration of IV vancomycin for 6 more weeks until cultures were negative and associated conditions resolved.
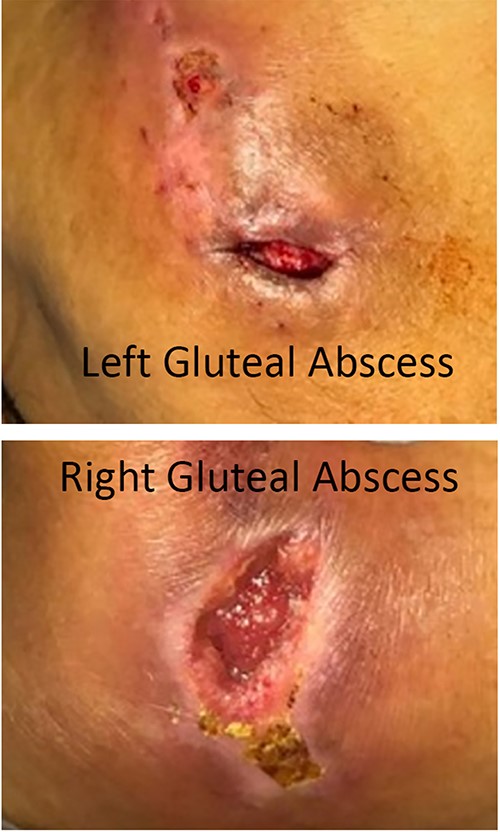
Digital photography of the patient’s untreated soft tissue infections taken during their initial emergency department presentation. (A) Image of the patient’s left gluteus demonstrating an open, draining abscess. (B) Image of the patient’s right gluteus demonstrating a second open, draining abscess.
After aqueous tap, intravitreal vancomycin and cephtazidime were administered along with topical antibiotic therapy. One week after presentation (Fig. 3A), pars plana vitrectomy was performed to debulk the exudation. In addition, retinal detachment that was noted secondary to necrosis at the time of surgery was treated with endolaser and placement of silicone oil.
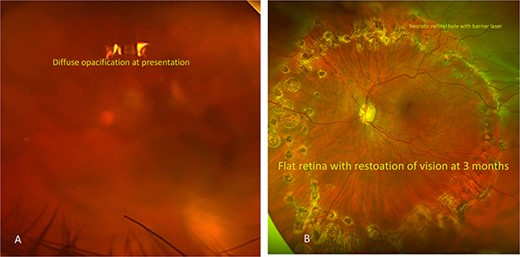
Color fundus photography of the patient’s left eye taken at different points during their disease course. (A) Fundus photography at the time of presentation showing diffuse vitritis with obscuration of details. (B) Fundus photography taken 3 months after hospital discharge demonstrating significant clearing of vitreous debris after PPV, RDR, vitreous debris removal and CEIOL. Barrier laser noted around necrotic retinal hole with flat retina.
Interval color fundus photography of the left eye taken at 3 months after the initial infection (Fig. 3B) demonstrated significant resolution of vitreous debris and BCVA improved from HM to 20/200.
DISCUSSION
EE occurs due to hematogenous seeding of microorganisms (bacterial or fungal) in the eye from increased blood flow to the retina, choroid and ciliary body. Unlike the more common exogenous endophthalmitis where damage is from toxins released from microorganisms, the damage in EE is from seeding of septic embolus in vitreous through choroidal/retinal circulation and associated inflammatory reaction [5]. Diabetes mellitus and associated vasculopathy are a commonly associated risk factor [3].
Skin or soft tissue infectious are an extremely rare extraocular foci of infection for EE. This is the first reported case of EE from soft tissue (gluteal abscesses) infection. Extensive literature review showed only two prior reported cases of EE associated with skin or soft tissue abscess. In both cases, endocarditis was the primary source of infection and soft tissue abscess was concurrently present but not the cause of EE [6, 7].
A systematic review of 342 cases of bacterial EE found that blood cultures were positive in only 56% of cases and intraocular sample was positive in only 58% of cases (comprising of 59% vitreous taps, 41% vitrectomy samples and 26% AC taps) [8]. Even with appropriate treatment, 44% of eyes had a visual acuity worse than 20/200, 24% of eyes required enucleation or evisceration and the mortality rate was 4% [8]. Poor prognosis may be the poor culture sensitivity with blood and intraocular samples and delay in implementing appropriate anti-microbial regimen. It is important to do a thorough review of systems in cases of EE so that other hematogenous sources of infections are not missed. Additional infections such as hepatic or renal abscess, appendageal vegetation were discovered during EE management [9].
Chronic non healing gluteal abscess (MRSA) was the nidus of infection for EE in our patient. Systemic septicemia and septic pulmonary emboli were successfully treated with prolonged IV antibiotics. The patient’s EE was successfully managed with aggressive intravitreal antibiotic injections along with topical therapy. Timely Pars plana vitrectomy (to evacuate vitreous abscess) and recognition and management of retinal detachment from associated necrotic retinal hole with endolaser resulted in good visual outcome (BCVA improved from HM to 20/200).
In summary, we describe a rare case of bacterial EE in an immunocompetent patient from bilateral gluteal abscess that was effectively managed (prompt topical and intravitreal antibiotics, pars plana vitrectomy) with successful recovery of vision.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



