-
PDF
- Split View
-
Views
-
Cite
Cite
Amy Edwards-Murphy, Helen Earley, Ben Creavin, Peter McCullough, Fiachra Cooke, Peter Neary, A fundamental change emerging in locally advanced rectal cancer management: a case report, Journal of Surgical Case Reports, Volume 2022, Issue 9, September 2022, rjac405, https://doi.org/10.1093/jscr/rjac405
Close - Share Icon Share
Abstract
Treatment of locally advanced rectal cancer remains a challenge in colorectal surgery. It has had an evolving landscape over the past three decades. Implementation of total neoadjuvant therapy (TNT) as a novel approach to management has begun globally but long-term outcomes and data analysis to identify optimal schedules are eagerly awaited. We report a case of locally advanced rectal cancer management in a young male with a complete pathological response to TNT.
INTRODUCTION
Although neoadjuvant chemoradiotherapy followed by surgery is the generally accepted standard of care for management of high-risk rectal cancer, there has been much debate on the optimal sequence of treatment [1]. Total neoadjuvant therapy (TNT) is an emerging treatment option for resectable, locally advanced rectal cancer (LARC).
This potential paradigm shift in practice comes with high levels of evidence from two randomized phase III trials within the past 18 months [2, 3]. With their proposed upfront approach to systemic treatment reported benefits include improved pathological response and reduced distant metastasis but also better compliance with chemotherapy and reduced toxicity. These trials demonstrate better both short- and long-term outcomes, which are promising in combating this difficult pathology.
Herein, we report a case of complete pathological response in a young male with locally advanced rectal cancer with bladder and right pelvic side wall invasion following TNT.
CASE
Our case is a 35-year-old male who presented with a short history of painless rectal bleeding on a background of significant weight loss over the preceding 12 months. Clinical examination demonstrated no abdominal mass or hepatomegaly and digital rectal examination normal. He was an otherwise healthy male, no past medical or surgical history and no regular medications. The patient was a nonsmoker, physically active, working full time and had no family history of colorectal cancer.
INVESTIGATIONS
He proceeded to sigmoidoscopy, which identified a neoplasm in the upper one-third of the rectum with evidence of luminal compromise. Biopsy of this lesion at index sigmoidoscopy confirmed moderately differentiated adenocarcinoma. No synchronous tumors were identified on completion colonoscopy. Carcinoembryonic antigen (CEA) level at diagnosis was 106 ng/mL. MR staging T4BN2Mx tumor with suggestion of local bladder invasion and right pelvic side wall (Fig. 1). Computerised tomography Thorax, Abdomen and Pelvis (CTTAP) demonstrated no distant metastasis in the liver or lung but did demonstrate para-aortic lymphadenopathy. Considering the lymphadenopathy and following Multidisciplinary team (MDT) discussion, a PET CT scan was arranged, which showed these to be uninvolved but did demonstrate positive inferior mesenteric artery nodes.
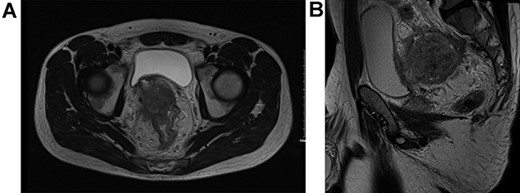
T4BN2MX Upper rectal cancer (A) axial view and (B) sagittal view.
TREATMENT
The patient underwent a defunctioning ileostomy at diagnosis. He completed 6 cycles of modified chemotherapy regime `FOLFIRINOX' (Folinic acid, fluorouracil, irinotecan and oaxliplatin) tolerated reasonably well, ECOG 0-1. He received Neulasta injections to prevent neutropenia during chemotherapy. This was followed by long course chemoradiotherapy (Capecitabine 825 mg/mtr2 twice daily, 5 days per week during radiotherapy). Repeat imaging 6 weeks post TNT demonstrated excellent response, reduction in bulky tumor previously noted on peritoneal reflection and bladder and extensive low signal scarring between rectum, peritoneal reflection, bladder and right sided internal iliac vessels (Fig. 2).
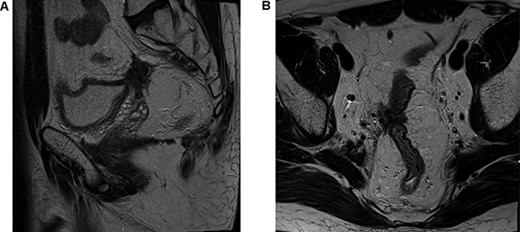
Post total neoadjuvant therapy (A) axial view and (B) sagittal view.
The patient proceeded to surgery 10 weeks after completion of TNT. Bilateral JJ stent insertion was arranged on the morning of surgery and the patient proceeded to an ultra-low anterior resection with partial cystectomy, obturator nodal bloc dissection, bladder repair with right ureteric reimplantation and reversal of ileostomy.
Outcome and follow up: The patient had an uncomplicated postoperative recovery and was discharged Day 10 post operatively with a urinary catheter in situ until urology were satisfied of no residual defect in the bladder wall on cystogram and cystoscopy. JJ stents were removed 6 weeks postoperatively at flexible cystoscopy. A small defect was visible on cystoscopy in the bladder wall and fluoroscopy confirmed a leak. The catheter remained for a further 6 weeks at which point fluoroscopy confirmed resolution and the catheter was successfully removed.
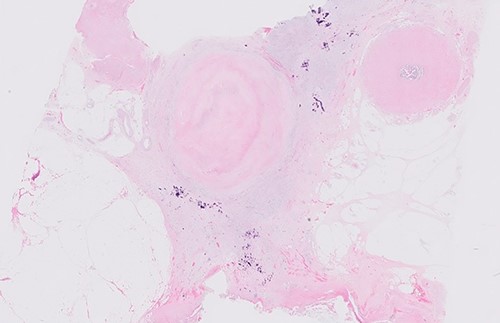
Postoperative histology demonstrated complete pathological response and an R0 resection (ypT0 N0(0/46) M0R0; Fig. 3). The patient proceeded to completion chemotherapy of 6 cycles of FOLFOX (folinic acid, fluorouracil and oxiliplatin) at 6 weeks postoperatively. He was then entered into a formal surveillance programme.
DISCUSSION
As the incidence of colorectal cancer in adults under 50 rises, there is widespread use of multimodality treatment strategies for locally advanced rectal cancer [4]. With a growing number of younger adults similar to our patient diagnosed with colorectal cancer, the optimal sequence of modalities in the management of resectable, locally advanced cancer is sought. This is a question addressed by recent trials, namely OPRA, RAPIDO and PRODIGE 23 [2, 3, 5]. Our patient received the treatment protocol described in the experimental arm of the PRODIGE 23 trial. This study showed significantly better outcomes for 3-year disease free survival, metastasis free survival, complete pathological response and adverse events in chemotherapy [3].
The pathological complete response (pCR) as seen in our case is recognized as a good prognostic indicator of overall survival as well as local and distant recurrence-free survival in comparison to those with residual viable disease. It is also recognized as one of the primary endpoints significantly improved upon in the PRODIGE 23 trial [3]. Our patient proceeded to definitive surgery 9 months after diagnosis and 10 weeks after completion of TNT. Time to surgery following completion of neoadjuvant therapy is a much-debated factor in the search for optimum care. In the case of our patient, repeat imaging post TNT noted extensive fibrotic changes within the surgical field, which can make the task of surgical excision even more difficult. Research suggests a wait of at least 10 weeks after treatment is best to achieve pCR, which is a goal of best management in colorectal cancer [6].
The combination of multivisceral surgical excision and radiotherapy generally achieve good local control. However, distant metastasis is one of the main modalities for failure in locally advanced rectal cancer [7]. Our patient did not have any distant metastasis at staging or at repeat imaging post TNT. This will be an area for close scrutiny going forward in patients now achieving pCR following TNT particularly in the early-onset colorectal cancer cohort.
An important milestone in our patient’s treatment was his post TNT imaging, which demonstrated an excellent response. It was an opportunity for defining anatomy and surgical planning. Magnetic resonance imaging (MRI) staging is key for evaluating response to treatment and specific parameters are available to tailor patient management based on factors such as sphincter involvement and pathological response on MRI [8].
TNT is a promising potential treatment option for LARC in patients eligible. This patient’s operative outcome lends support to the reported positive outcomes post TNT approach.
CONFLICT OF INTEREST STATEMENT
The authors confirm no conflict of interest.
FUNDING
No funding was received for this case report.
CONSENT
Informed written consent was obtained from the patient involved and every effort was made to ensure anonymity of the patient.
References
Bahadoer, Renu R., et al. .
Conroy T, et al.
Goffredo P, et al.



