-
PDF
- Split View
-
Views
-
Cite
Cite
Danah F Alrusayyis, Abdulaziz K Alaraifi, Salwa Alhumaid, Abdul Latif Khan, Mohammed Elkrim, Late presentation of laryngeal lipoid proteinosis: a case report and review of the literature, Journal of Surgical Case Reports, Volume 2022, Issue 8, August 2022, rjac360, https://doi.org/10.1093/jscr/rjac360
Close - Share Icon Share
Abstract
Lipoid proteinosis (LP) is a rare autosomal-recessive disease characterized by the deposition of hyaline material infiltrating multiple body organs, including the larynx. The possible clinical presentations are wide-ranging. Laryngeal involvement results in dysphonia that is noted at birth or infantile years. The diagnosis is based on histological findings, and the management options vary depending on the severity of the symptoms. In this paper, we report an unusual case of LP with laryngeal involvement in an adult patient, along with a review of current literature.
INTRODUCTION
Lipoid proteinosis (LP) was first termed ‘lipoidosis cutis et mucosae’ in 1929 by the dermatologist Erich Urbach and the otolaryngologist Camillo Wiethe [1]. Since then, over 300 case reports have been published [2]. It is an autosomal recessive disease caused by mutations in the extracellular matrix protein 1 (ECM1) gene located on chromosome 1q21 [3]. This leads to the accumulation of an amorphous hyaline substance in the skin, eyelids and mucosal tissues, with a prime focus on the upper aerodigestive tract [4]. Although cutaneous lesions manifest in the first 2 years of life, the hoarse cry at birth due to hyaline deposits in the larynx makes earlier diagnosis possible [5]. In this paper, we report an unusual case of LP with laryngeal involvement in an adult patient, along with a review of current literature.
CASE REPORT
A 53-year-old female, known to have bronchial asthma and diabetes mellitus, was referred to the otolaryngology clinic for a long-standing dysphonia. She is a housewife and non-smoker. Her dysphonia was noticed 15 years ago and was associated with intermittent dysphagia to solid only. She did not have airway symptoms, systemic manifestations or constitutional symptoms.
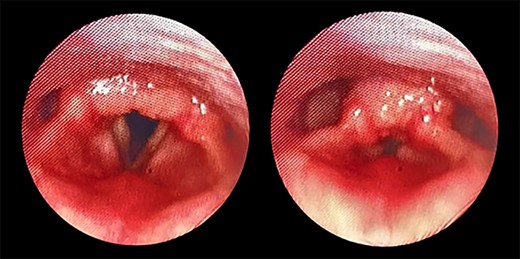
Transnasal flexible endoscope showing broad-based lesion affecting the middle two-third of the right vocal fold with a granular appearance of the interarytenoid region.
The patient maintained good oxygen saturation levels in room air with no signs of respiratory distress. She had a hoarse voice with a maximum phonation time of 10 s. Neck examination was unremarkable. Bedside transnasal flexible endoscope revealed bilateral vocal folds movement with polypoid lesions involving the middle two-thirds of the right vocal fold and the posterior part of the left vocal fold with edematous interarytenoid mucosa. Moreover, polypoid lesions were also seen in the base of tongue, epiglottis, with cobblestoning of the posterior pharyngeal wall (Figs 1 and 2). Basic laboratory tests were within the normal range. A contrasted neck computed tomography (CT) scan revealed bilateral asymmetric thickening of the vocal folds with medialization of the right vocal fold, with no enlarged cervical lymph nodes (Fig. 3).
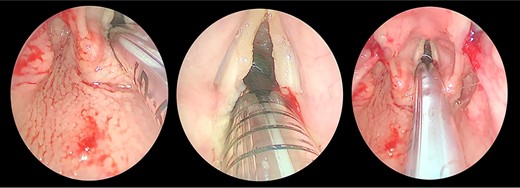
Direct laryngobronchoscopy showing a polypoidal lesion on the middle third of the left vocal fold with a granular appearance of the interarytenoid region and cobblestoning of the posterior pharyngeal wall.
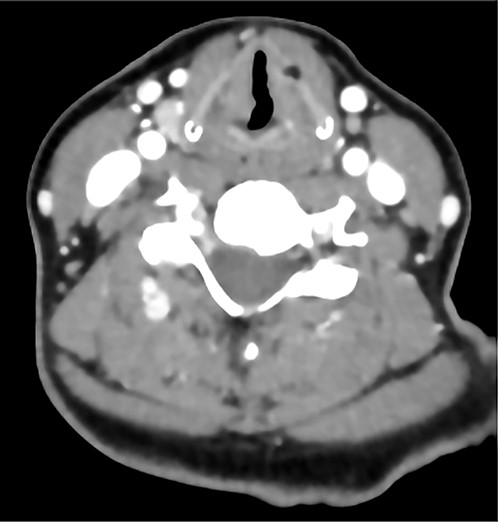
A contrasted neck CT scan showing bilateral asymmetric thickening of the vocal folds with medialization of the right vocal fold.
The patient underwent direct microlaryngoscopy (MLS) with multiple biopsies under general anesthesia. MLS revealed the same previous findings with a clear subglottic area (Figs 1 and 2). Multiple biopsies were obtained from the mentioned regions using punch forceps. There were no intraoperative or postoperative complications. The patient was discharged in stable condition the following day.
Histopathology showed abundant deposits of acellular amorphous hyaline material in the subepithelial stroma. This material was diastase-resistant Periodic acid–Schiff (PAS) positive, and stained with Alcian blue (pH 2.5) and Elastin van Gieson (EVG). It was negative for Congo Red (amyloid) and Trichrome stain (for fibrin). The overall features were consistent with LP (Fig. 4). No clinically relevant variants were identified in the ECM1 gene by sequence analysis. The patient was referred to internal medicine to rule out the systemic manifestations of the disease; their investigations were unremarkable. The patient was given the options of surgical and medical management; she preferred medical management. She was managed conservatively with an anti-reflux medication, oral corticosteroids for 14 days and postoperative voice rest instructions. She was reassessed 3 and 6 months postoperatively with stable laryngeal symptoms and resolved dysphagia and was given a yearly follow-up for reassessment.
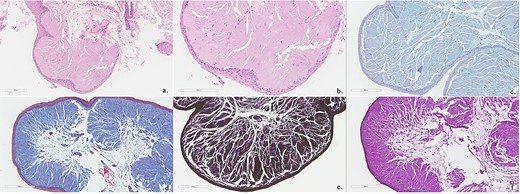
Biopsies show polypoid squamous epithelium-lined stroma containing abundant amorphous, eosinophilic acellular hyaline material (Hematoxylin and Eosin stain; a, b). This material is Alcian blue positive (c), PASD positive (d), EVG positive (e) and Trichrome (for fibrin) negative (f).
DISCUSSION
The prevalence of the disease increases in areas where consanguinity is common, such as Saudi Arabia. Although the symptoms are usually noted at birth or infantile years [6], the dysphonia of our 53-year-old patient manifested just 15 years ago. Therefore, this unusual presentation adds to the rarity of the disease.
Dysphonia is a classic symptom that presents in two-thirds of patients. It manifests as a weak cry in newborns or the first years of life [4, 5, 7]. The gradual loss of vocal cords elasticity worsens the symptoms, which explains the late presentation of some patients [4, 5]. Complicated cases may develop total aphonia [2]. Therefore, otolaryngologists must add LP to their differential diagnosis of dysphonia [5, 8, 9]. Other significant head and neck findings include white-yellow mucosal plaques or papules and dental impressions on the tongue [5, 6]. Moreover, patients may complain of recurrent swelling of salivary glands, particularly the submandibular and parotid glands, due to ductal stenosis [10]. Xerostomia, poor oral hygiene, parotitis and parotid fistula may become evident [11].
Life-threatening conditions related to airway compromise are rarely witnessed in LP patients. Tongue hypertrophy and thickening of the sublingual frenulum can result in difficulty protruding the tongue and dysphagia [6]. Deposits may also involve the epiglottis, uvula, tonsils and oral mucosa. Respiratory distress can occur after a diffuse infiltration of the pharynx and larynx, necessitating tracheostomy [4, 8]. Contrasting to our patient, incomplete glottal closure and restricted vocal fold mobility were evident in some patients with severe hoarseness [5, 12].
Although not present in our case, dermatological manifestations are common. In addition, moniliform blepharosis, a flesh-colored string of beaded papules on eyelid margins, are a pathognomonic finding described in about 50% of cases [2, 4, 5, 13]. Intracranial calcifications may be seen in more complicated cases. Epilepsy, memory deficits, mental retardation, personality changes and schizophrenic behavior are common neurological presentations [6, 8, 14].
Histopathological examination of mucocutaneous lesions is the ultimate approach for diagnosis [3]. Microscopic analysis of eosinophilic, PAS-positive, diastase-resistant material in the submucosal layer of the dermis is a hallmark of LP [5, 8]. Examination of skin biopsy specimens shows thickening of the basement membrane, dermo-epidermal junction, papillary dermis, adjacent capillaries and around adnexal epithelia [2, 13]. Our patient underwent laryngeal biopsies only, which showed abundant subepithelial deposits of acellular amorphous material, a consistent finding of LP [5]. Brain CT and magnetic resonance imaging studies may show calcifications in the hippocampus and temporal lobes in later stages of the disease [6, 8, 14].
The effectiveness of therapeutic approaches is still questionable. MLS with CO2 laser ablation showed clinical improvement in laryngeal symptoms but can cause scarring and stenosis. Surgical approaches are also avoided due to associated complications and morbidity. However, tracheostomy is preserved for patients with complete airway obstruction [4, 6]. Oral acitretin, etretinate, dimethyl sulfoxide, corticosteroids and methotrexate are recently reported symptom relievers [5]. Other patients may require intensive treatment like dermabrasion and chemical skin peeling [3].
LP is a chronic disease with no identified cure, yet it is rarely considered life-threatening [11]. It does not affect patients’ life expectancy even after performing critical procedures if prevention and early identification of complications are considered [3].
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
FUNDING
No funding was received for this study.



