-
PDF
- Split View
-
Views
-
Cite
Cite
Rasha T Kakati, Walid Faraj, Taha Qaraqe, Frederic El Chaer, Hero Hussain, Ali Shamseddine, Mohamad Jawad Khalife, Immunotherapy for metastatic liver disease from colorectal carcinoma: case series from the Middle East, Journal of Surgical Case Reports, Volume 2022, Issue 8, August 2022, rjac142, https://doi.org/10.1093/jscr/rjac142
Close - Share Icon Share
Abstract
Immunotherapy poses new considerations and alterations to the management of metastatic colorectal carcinoma (mCRC), where chemotherapy achieves complete radiological response but yields complete pathological response in few patients only. Immunotherapy may be superior in the conversion of unresectable disease to resectable liver lesions from mCRC and downsizing borderline lesions for more feasible resectability and achieving complete pathologic response, with the potential for cure and to alter current, established guidelines for surgical resection with a shift from chemotherapy. We present two patients with hepatic lesions from mCRC characterized by deficient mismatch repair (dMMR) which were unresectable after traditional chemotherapy but were converted to resectable lesions with a complete histopathological response following immunotherapy. Complete histopathologic response and radiologic regression or disappearance of liver lesions was observed in patients with dMMR mCRC after pembrolizumab. Immunotherapy exhibits notable potential for cure, achieving complete, successful surgical resection and improving prognosis.
INTRODUCTION
Colorectal carcinoma (CRC) is the third most commonly occurring cancer in males and the second in females; the liver is the most common metastatic organ for CRC [1, 2]. Stangl et al. reported a median survival of 31, 7.9, 2.6 and 0.9 at 1, 2, 3 and 4 years, respectively, for patients with metastatic colorectal carcinoma (mCRC); even with chemotherapy, the 5-year survival rates remain low at around 9% [3].
Surgical resection remains the gold standard for metastatic liver lesions in mCRC while improving survival rates [4]. Current guidelines suggest that any resectable tumor should be resected with a complete (R0) resection intention with at least a 20% functional residual volume [5–7].
As initial curative resection is not always possible, patients with unresectable liver lesions require chemotherapy to downsize those lesions and control micrometastases, hence converting 15% of the 10–25% of patients with initially unresectable disease to resectable candidates [5, 8, 9]. Complete response is observed in only 9% of patients receiving chemotherapy [10].
The advancement in in targeted therapies, mainly immunotherapy, provides potential for improving response, achieving complete surgical resection and achieving better survival rates. Microsatellite instability-high (MSI-H) mCRC occurs due to mutations in mismatch repair (MMR) genes, leading to silenced or malfunctioning of the MMR proteins, including MutL Homolog 1 (MLH1), MutS Homolog 2 (MSH2), MutS Homolog 6 (MSH6) and PMS1 Homolog 2 (PMS2), which normally complex to recognize mismatch and guide repair, yielding deficient mismatch repair (dMMR) tumors [1, 11, 12]. Tumor cells express Programmed Death-Ligand 1 (PD-L1) which, when recognized by Programmed Death-1 on cytotoxic T-cells, blocks the immune response, allowing perpetuation of cancer cells [13]. Blockade with anti-PD-L1 antibodies, such as pembrolizumab, has thus been considered to overcome dMMR tumor cells, with promising results shown with various cancers [13]. Intermittent results from the Phase II KEYNOTE-016 and CHECKMATE 142 trials culminated in the approval and advocacy for the use of pembrolizumab by the National Comprehensive Cancer Network and the Food and Drug Administration (FDA) since 2017 for recurrent or mCRC, which is MSI-H/dMMR [1, 14]. Notably, KEYNOTE-177, a new Phase III randomized controlled trial investigating the efficacy and safety of pembrolizumab, was launched in 2015 with early suggestions for superiority as first-line therapy with improved progression-free survival (PFS) at 16.5 months compared to 8.2 months with chemotherapy [1, 15].
In light of this, immunotherapy shows very promising results in patients with mCRC; in particular, pembrolizumab can convert unresectable disease, downsize borderline lesions and achieve R0 resection after surgery. We are reporting a case series of two patients who showed complete histopathological response after immunotherapy.
CASE PRESENTATION
First case
A 33-year-old female patient was diagnosed with a moderately differentiated invasive adenocarcinoma of the transverse colon with multiple bilobar liver lesions (Fig. 1A1 and Table 1). She underwent a laparoscopic subtotal colectomy, and the immunohistochemical analysis showed loss of MLH1 and PMS2 expression in tumor cells with retained MSH2 and MSH6, thus MMR-deficient (dMMR). She was started on FOLFOX (Oxaliplatin with 5-Fluorouracyl and Leucovorin) regimen with Avastin (Bevacizumab). Computed tomography (CT) scan was performed after her fourth cycle of chemotherapy and revealed an increase in size of the liver lesions (Table 1). She was started on pembrolizumab (19 cycles), and upon follow-up CT scans done every 3 months, there was a significant decrease in the size of the hepatic lesions (Fig. 1A2). She was reassessed and underwent liver resections of all her lesions. Final pathology was negative for residual carcinoma, with areas of necrosis and negative margins on surgical pathology.
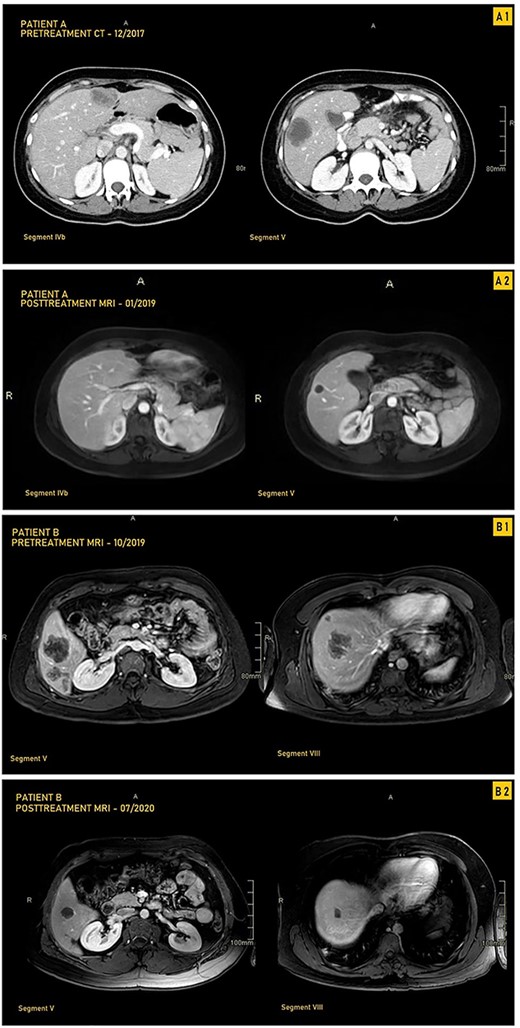
CT and MRI images our two patients before and after treatment with pembrolizumab; (A1) Pretreatment MRI of our first patient in September 2017 showing most prominent lesions, located in Segments IVb and V, on axial enhanced CT after contrast administration; (A2) Posttreatment axial enhanced T1-weighted MRI of our first patient in January 2019 showing most prominent lesions located in Segments IVb and V; the lesion in Segment V is smaller, measuring 1.4 cm, and is completely necrotic; (B1) Pretreatment axial enhanced T1-weighted sequence MRI performed on 3Tesla field strength following gadolinium contrast administration in our first patient in October 2019 showing most prominent lesions, located in Segments V and VIII; the largest metastatic lesion is a 4.8-cm heterogeneously enhancing metastatic lesion in Segment VIII; (B2) Posttreatment axial enhanced T1-weighted sequence MRI performed on 3Tesla field strength following gadolinium contrast administration in the hepatobiliary phase in our second patient in July 2020, showing most prominent lesions located in Segments V and VIII; the largest lesion in Segment VIII decreases in size to 1.5 cm following treatment and is completely necrotic.
Progression of size and response as noted on CT and MRI of hepatic metastatic lesions and the colonic mass at different points of the treatment course in our two patients
| Imaging date . | Treatment stage . | Segment/location . | No. of lesions per segment . | Size of largest lesion (cm) . | Response . |
|---|---|---|---|---|---|
| First patient | |||||
| September 2017 | Initial presentation prior to therapy initiation | Segment I | None | - | Baseline |
| Segment II | 1 | 1.7 × 1.5 | |||
| Segment III | None | - | |||
| Segment IVa | None | - | |||
| Segment IVb | 1 | 2.1 × 1.7 | |||
| Segment V | 2 | 1.6 × 1.5 | |||
| Segment VI | None | - | |||
| Segment VII | 1 | 1.1 × 0.7 | |||
| Segment VIII | None | - | |||
| Colon | 1 | 6.2 × 4.1 × 3.8 | |||
| December 2017 | After subtotal colectomy, Folfox and Avastin | Segment I | None | - | - |
| Segment II | 1 | 3.4 × 3 | Increase in size | ||
| Segment III | None | - | - | ||
| Segment IVa | None | - | - | ||
| Segment IVb | 1 | 4 × 2.5 | Increase in size | ||
| Segment V | 2 | 4.1 × 3.4 and smaller 2.2 × 1.8 | |||
| Segment VI | None | - | - | ||
| Segment VII | 1 | 0.9 × 0.8 | Decrease in size | ||
| Segment VIII | None | - | - | ||
| Colon | None | - | Resected | ||
| January 2019 | After 19 cycles of pembrolizumab and prior to metastasectomy | Segment I | None | - | - |
| Segment II | 1 | 0.9 × 0.8 | Decrease in size | ||
| Segment III | None | - | - | ||
| Segment IVa | None | - | - | ||
| Segment IVb | 1 | 1.4 × 0.9 | Decrease in size | ||
| Segment V | 2 | 1.4 × 1.3 and smaller 0.7 × 0.6 | Decrease in size | ||
| Segment VI | None | - | - | ||
| Segment VII | 1 | 0.5 × 4 | Decrease in size | ||
| Segment VIII | None | - | - | ||
| Colon | None | - | Resected | ||
| Second patient | |||||
| October 2019 | After receiving four cycles of Xelox and Avastin | Segment I | 1 | 1.9 × 1.2 | Baseline |
| Segment II | 2 | 2.7 × 2.1 | |||
| Segment III | None | - | |||
| Segment IVa | 1 | 1 × 0.9 | |||
| Segment IVb | None | - | |||
| Segment V | 5 | 4.7 × 4.5 | |||
| Segment VI | 5 | 4.2 × 3.7 | |||
| Segment VII | 4 | 1.7 × 1.3 | |||
| Segment VIII | 6 | 4.8 × 4.1 | |||
| Colon | 1 | 4.5 × 2.9 | |||
| July 2020 | After 12 cycles of pembrolizumab and prior to metastasectomy | Segment I | 0 | - | Zero of one—lesion no longer seen |
| Segment II | 2 | 1.1 × 0.7 | Decrease in size | ||
| Segment III | None | - | - | ||
| Segment IVa | 1 | 0.4 × 0.4 | Decrease in size | ||
| Segment IVb | None | - | - | ||
| Segment V | 4 | 2.2 × 1.9 | Four of five lesions—one lesion no longer seen, four decreased in size | ||
| Segment VI | 5 | 1.3 × 1 | Decrease in size | ||
| Segment VII | 4 | 0.3 | Decrease in size | ||
| Segment VIII | 4 | 1.5 × 1.2 | Four of six lesions—two lesions no longer seen, four lesions decreased in size | ||
| Colon | 1 | 1.7 × 1.5 | Decrease in size | ||
| Imaging date . | Treatment stage . | Segment/location . | No. of lesions per segment . | Size of largest lesion (cm) . | Response . |
|---|---|---|---|---|---|
| First patient | |||||
| September 2017 | Initial presentation prior to therapy initiation | Segment I | None | - | Baseline |
| Segment II | 1 | 1.7 × 1.5 | |||
| Segment III | None | - | |||
| Segment IVa | None | - | |||
| Segment IVb | 1 | 2.1 × 1.7 | |||
| Segment V | 2 | 1.6 × 1.5 | |||
| Segment VI | None | - | |||
| Segment VII | 1 | 1.1 × 0.7 | |||
| Segment VIII | None | - | |||
| Colon | 1 | 6.2 × 4.1 × 3.8 | |||
| December 2017 | After subtotal colectomy, Folfox and Avastin | Segment I | None | - | - |
| Segment II | 1 | 3.4 × 3 | Increase in size | ||
| Segment III | None | - | - | ||
| Segment IVa | None | - | - | ||
| Segment IVb | 1 | 4 × 2.5 | Increase in size | ||
| Segment V | 2 | 4.1 × 3.4 and smaller 2.2 × 1.8 | |||
| Segment VI | None | - | - | ||
| Segment VII | 1 | 0.9 × 0.8 | Decrease in size | ||
| Segment VIII | None | - | - | ||
| Colon | None | - | Resected | ||
| January 2019 | After 19 cycles of pembrolizumab and prior to metastasectomy | Segment I | None | - | - |
| Segment II | 1 | 0.9 × 0.8 | Decrease in size | ||
| Segment III | None | - | - | ||
| Segment IVa | None | - | - | ||
| Segment IVb | 1 | 1.4 × 0.9 | Decrease in size | ||
| Segment V | 2 | 1.4 × 1.3 and smaller 0.7 × 0.6 | Decrease in size | ||
| Segment VI | None | - | - | ||
| Segment VII | 1 | 0.5 × 4 | Decrease in size | ||
| Segment VIII | None | - | - | ||
| Colon | None | - | Resected | ||
| Second patient | |||||
| October 2019 | After receiving four cycles of Xelox and Avastin | Segment I | 1 | 1.9 × 1.2 | Baseline |
| Segment II | 2 | 2.7 × 2.1 | |||
| Segment III | None | - | |||
| Segment IVa | 1 | 1 × 0.9 | |||
| Segment IVb | None | - | |||
| Segment V | 5 | 4.7 × 4.5 | |||
| Segment VI | 5 | 4.2 × 3.7 | |||
| Segment VII | 4 | 1.7 × 1.3 | |||
| Segment VIII | 6 | 4.8 × 4.1 | |||
| Colon | 1 | 4.5 × 2.9 | |||
| July 2020 | After 12 cycles of pembrolizumab and prior to metastasectomy | Segment I | 0 | - | Zero of one—lesion no longer seen |
| Segment II | 2 | 1.1 × 0.7 | Decrease in size | ||
| Segment III | None | - | - | ||
| Segment IVa | 1 | 0.4 × 0.4 | Decrease in size | ||
| Segment IVb | None | - | - | ||
| Segment V | 4 | 2.2 × 1.9 | Four of five lesions—one lesion no longer seen, four decreased in size | ||
| Segment VI | 5 | 1.3 × 1 | Decrease in size | ||
| Segment VII | 4 | 0.3 | Decrease in size | ||
| Segment VIII | 4 | 1.5 × 1.2 | Four of six lesions—two lesions no longer seen, four lesions decreased in size | ||
| Colon | 1 | 1.7 × 1.5 | Decrease in size | ||
Progression of size and response as noted on CT and MRI of hepatic metastatic lesions and the colonic mass at different points of the treatment course in our two patients
| Imaging date . | Treatment stage . | Segment/location . | No. of lesions per segment . | Size of largest lesion (cm) . | Response . |
|---|---|---|---|---|---|
| First patient | |||||
| September 2017 | Initial presentation prior to therapy initiation | Segment I | None | - | Baseline |
| Segment II | 1 | 1.7 × 1.5 | |||
| Segment III | None | - | |||
| Segment IVa | None | - | |||
| Segment IVb | 1 | 2.1 × 1.7 | |||
| Segment V | 2 | 1.6 × 1.5 | |||
| Segment VI | None | - | |||
| Segment VII | 1 | 1.1 × 0.7 | |||
| Segment VIII | None | - | |||
| Colon | 1 | 6.2 × 4.1 × 3.8 | |||
| December 2017 | After subtotal colectomy, Folfox and Avastin | Segment I | None | - | - |
| Segment II | 1 | 3.4 × 3 | Increase in size | ||
| Segment III | None | - | - | ||
| Segment IVa | None | - | - | ||
| Segment IVb | 1 | 4 × 2.5 | Increase in size | ||
| Segment V | 2 | 4.1 × 3.4 and smaller 2.2 × 1.8 | |||
| Segment VI | None | - | - | ||
| Segment VII | 1 | 0.9 × 0.8 | Decrease in size | ||
| Segment VIII | None | - | - | ||
| Colon | None | - | Resected | ||
| January 2019 | After 19 cycles of pembrolizumab and prior to metastasectomy | Segment I | None | - | - |
| Segment II | 1 | 0.9 × 0.8 | Decrease in size | ||
| Segment III | None | - | - | ||
| Segment IVa | None | - | - | ||
| Segment IVb | 1 | 1.4 × 0.9 | Decrease in size | ||
| Segment V | 2 | 1.4 × 1.3 and smaller 0.7 × 0.6 | Decrease in size | ||
| Segment VI | None | - | - | ||
| Segment VII | 1 | 0.5 × 4 | Decrease in size | ||
| Segment VIII | None | - | - | ||
| Colon | None | - | Resected | ||
| Second patient | |||||
| October 2019 | After receiving four cycles of Xelox and Avastin | Segment I | 1 | 1.9 × 1.2 | Baseline |
| Segment II | 2 | 2.7 × 2.1 | |||
| Segment III | None | - | |||
| Segment IVa | 1 | 1 × 0.9 | |||
| Segment IVb | None | - | |||
| Segment V | 5 | 4.7 × 4.5 | |||
| Segment VI | 5 | 4.2 × 3.7 | |||
| Segment VII | 4 | 1.7 × 1.3 | |||
| Segment VIII | 6 | 4.8 × 4.1 | |||
| Colon | 1 | 4.5 × 2.9 | |||
| July 2020 | After 12 cycles of pembrolizumab and prior to metastasectomy | Segment I | 0 | - | Zero of one—lesion no longer seen |
| Segment II | 2 | 1.1 × 0.7 | Decrease in size | ||
| Segment III | None | - | - | ||
| Segment IVa | 1 | 0.4 × 0.4 | Decrease in size | ||
| Segment IVb | None | - | - | ||
| Segment V | 4 | 2.2 × 1.9 | Four of five lesions—one lesion no longer seen, four decreased in size | ||
| Segment VI | 5 | 1.3 × 1 | Decrease in size | ||
| Segment VII | 4 | 0.3 | Decrease in size | ||
| Segment VIII | 4 | 1.5 × 1.2 | Four of six lesions—two lesions no longer seen, four lesions decreased in size | ||
| Colon | 1 | 1.7 × 1.5 | Decrease in size | ||
| Imaging date . | Treatment stage . | Segment/location . | No. of lesions per segment . | Size of largest lesion (cm) . | Response . |
|---|---|---|---|---|---|
| First patient | |||||
| September 2017 | Initial presentation prior to therapy initiation | Segment I | None | - | Baseline |
| Segment II | 1 | 1.7 × 1.5 | |||
| Segment III | None | - | |||
| Segment IVa | None | - | |||
| Segment IVb | 1 | 2.1 × 1.7 | |||
| Segment V | 2 | 1.6 × 1.5 | |||
| Segment VI | None | - | |||
| Segment VII | 1 | 1.1 × 0.7 | |||
| Segment VIII | None | - | |||
| Colon | 1 | 6.2 × 4.1 × 3.8 | |||
| December 2017 | After subtotal colectomy, Folfox and Avastin | Segment I | None | - | - |
| Segment II | 1 | 3.4 × 3 | Increase in size | ||
| Segment III | None | - | - | ||
| Segment IVa | None | - | - | ||
| Segment IVb | 1 | 4 × 2.5 | Increase in size | ||
| Segment V | 2 | 4.1 × 3.4 and smaller 2.2 × 1.8 | |||
| Segment VI | None | - | - | ||
| Segment VII | 1 | 0.9 × 0.8 | Decrease in size | ||
| Segment VIII | None | - | - | ||
| Colon | None | - | Resected | ||
| January 2019 | After 19 cycles of pembrolizumab and prior to metastasectomy | Segment I | None | - | - |
| Segment II | 1 | 0.9 × 0.8 | Decrease in size | ||
| Segment III | None | - | - | ||
| Segment IVa | None | - | - | ||
| Segment IVb | 1 | 1.4 × 0.9 | Decrease in size | ||
| Segment V | 2 | 1.4 × 1.3 and smaller 0.7 × 0.6 | Decrease in size | ||
| Segment VI | None | - | - | ||
| Segment VII | 1 | 0.5 × 4 | Decrease in size | ||
| Segment VIII | None | - | - | ||
| Colon | None | - | Resected | ||
| Second patient | |||||
| October 2019 | After receiving four cycles of Xelox and Avastin | Segment I | 1 | 1.9 × 1.2 | Baseline |
| Segment II | 2 | 2.7 × 2.1 | |||
| Segment III | None | - | |||
| Segment IVa | 1 | 1 × 0.9 | |||
| Segment IVb | None | - | |||
| Segment V | 5 | 4.7 × 4.5 | |||
| Segment VI | 5 | 4.2 × 3.7 | |||
| Segment VII | 4 | 1.7 × 1.3 | |||
| Segment VIII | 6 | 4.8 × 4.1 | |||
| Colon | 1 | 4.5 × 2.9 | |||
| July 2020 | After 12 cycles of pembrolizumab and prior to metastasectomy | Segment I | 0 | - | Zero of one—lesion no longer seen |
| Segment II | 2 | 1.1 × 0.7 | Decrease in size | ||
| Segment III | None | - | - | ||
| Segment IVa | 1 | 0.4 × 0.4 | Decrease in size | ||
| Segment IVb | None | - | - | ||
| Segment V | 4 | 2.2 × 1.9 | Four of five lesions—one lesion no longer seen, four decreased in size | ||
| Segment VI | 5 | 1.3 × 1 | Decrease in size | ||
| Segment VII | 4 | 0.3 | Decrease in size | ||
| Segment VIII | 4 | 1.5 × 1.2 | Four of six lesions—two lesions no longer seen, four lesions decreased in size | ||
| Colon | 1 | 1.7 × 1.5 | Decrease in size | ||
Second case
A 33-year-old male patient was diagnosed with adenocarcinoma of the transverse colon with 24 bilobar hepatic metastases. He was started on four cycles of Xelox and Avastin after which some lesions responded to treatment while others did not, as evidenced by changes in the hepatic lesions on magnetic resonance imaging (MRI) (Fig. 1B1 and Table 1). Immunohistochemical analysis showed loss of MLH1 and PMS2 with retention of MSH2 AND MSH6; he was started on pembrolizumab (12 cycles) with consistent interval decrease in size of both the colonic mass and hepatic lesions over several follow-ups every 3 months (Table 1). After multiple months, MRI showed a marked decrease in the transverse colonic mass and all hepatic lesions which became necrotic; four hepatic lesions became barely visible or disappeared (Table 1 and Fig. 1B2). He underwent a transverse colectomy with primary anastomosis and metastasectomy where all hepatic lesions were negative for residual carcinoma with negative surgical margins and areas of necrosis.
DISCUSSION
In light of the high occurrence of liver metastases among patients with mCRC, achieving complete histopathologic response and a successful surgical cure is essential. Where chemotherapy provides questionable resectability changes for hepatic lesions, the necessity for improved response preoperatively is essential for improved clinical course, quality of life and survival. Two patients were diagnosed with dMMR colorectal carcinoma metastatic liver lesions.
Despite receiving prior chemotherapy which imparted an overall increase in the size of the metastatic hepatic lesions in our second patient, pembrolizumab demonstrated efficacy in reducing all lesions; this was also observed in our first patient when used as first-line treatment (Fig. 2). Neither patient reported adverse effects; they both experienced a smooth clinical course during follow-up.
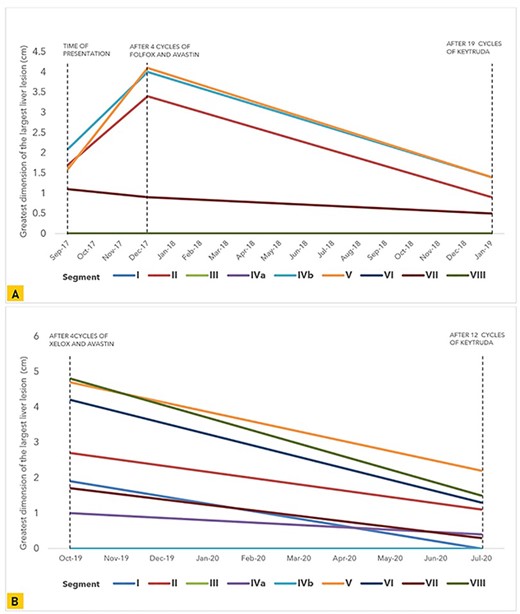
Graphical representation of the trend in size of the greatest tumor dimension of the largest liver lesions noted on CT and MRI in each liver segment at multiple points during the course of treatment in (A) our first patient and (B) our second patient.
Among both patients, complete histopathological response was demonstrated after surgery in all resected hepatic specimens. Multiple considerations surface regarding the potential of immunotherapy to modify pre-surgical management to ensure complete response and successful surgical resection with among this patient population. We demonstrate that immunotherapy poses a potential to achieve both complete histopathologic and radiologic responses.
The concept of resectable disease has potential to evolve with this novel treatment strategy; patients with hepatic lesions considered impossible to resect can now be considered as candidates for successful surgical management and cure from CRC. These considerations, in addition to surgical experience and the rise in molecular diagnostic techniques, have the potential to revise and impart modifications on currently established principles guiding pre-surgical management to attain effective surgical resection.
In line with our findings, in June 2020, the FDA approved pembolizumab for the first-line treatment of patients with unresectable or metastatic MSI-H/dMMR CRC due to ongoing KEYNOTE-177 results. Furthermore, in December 2020, official KEYNOTE-177 findings were released, demonstrating a 2-fold increase in PFS among patients receiving first-line pembrolizumab, confirming and enhancing our findings alongside [16]. Immunotherapy can change the concept of surgical management of patients with mCRC to the liver, not only by improving PFS but also by achieving complete histopathologic response and attaining cure. We envision notable curative potential and recommend clinical trials investigating histopathologic response to pembrolizumab in a large sample of patients with mCRC to the liver.
CONCLUSION
Complete histopathologic response of liver lesions with pembrolizumab in patients with dMMR mCRC exhibits notable potential for cure and achieving complete, successful surgical resection. We demonstrate 100% histopathologic response as well as radiologic regression or disappearance in 29 lesions among one patient receiving first-line pembrolizumab and another patient receiving pembrolizumab after progression with chemotherapy. This poses notable considerations to further investigate pembrolizumab as a novel treatment strategy with curative potential confirmed after resection to improve prognosis.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
CONSENT FOR PUBLICATION
Both patients provided consent to publish these findings.
References
Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J.
Wilson SM. Surgical treatment of hepatic metastases from colorectal cancers.
Khatri VP, Petrelli NJ, Belghiti J.
Author notes
A. Shamseddine and M. J. Khalife are Senior Researchers.



