-
PDF
- Split View
-
Views
-
Cite
Cite
Takahiko Sakuma, Ayaka Yokoi, Shu Ichihara, Appendiceal metastasis of gastric cancer clinically masquerading acute appendicitis: possible route of metastasis, Journal of Surgical Case Reports, Volume 2022, Issue 7, July 2022, rjac322, https://doi.org/10.1093/jscr/rjac322
Close - Share Icon Share
Abstract
Cancer metastasis to appendix vermiformis is rare. We here report a case of appendiceal metastasis of gastric cancer, which was incidentally discovered in the appendix resected as acute appendicitis. A 65-year-old man, who had undergone distal gastrectomy for poorly differentiated adenocarcinoma 2 years before, complained of lower abdominal pain. Physical examination and laboratory tests clinically suggested acute appendicitis. Macroscopically, the serosal surface of the resected appendix was hyperaemic and white-coated. These findings were compatible with the clinical diagnosis. However, histological examination revealed intra-mural invasion of poorly differentiated adenocarcinoma. The appendix serosal and mucosal surfaces were spared from cancer involvement. As the morphological appearance of adenocarcinoma and associated extensive lymphatic invasion was similar to those seen in the primary gastric cancer, the adenocarcinoma observed in the appendix was diagnosed as a metastasis. Possible routes of metastasis to the appendix from stomach were discussed with a brief review of relevant literature.
INTRODUCTION
Metastasis of cancer to appendix vermiformis is uncommon. Most of them develop as peritoneal dissemination in the terminal stage of cancer. A small number of cases of solitary appendiceal metastases from various primary cancers have been sporadically described often as a single case report. We here report a case of appendiceal metastasis from gastric cancer, in which the route of metastasis was thought as via lymphatic channels.
CASE REPORT
A 65-year-old male presented with pain in the right lower abdominal quadrant. Pain/tenderness in the right lower quadrant abdomen and elevated serum C-reactive protein suggested acute appendicitis. Appendectomy by conventional laparotomy was performed. Macroscopically, the appendix vermiformis was hyperaemic and covered with white coat (Fig. 1A), which was compatible with the clinical diagnosis. Unexpectedly, histologic examination demonstrated poorly differentiated adenocarcinoma along with phlegmonous appendicitis. Beneath the lamina muscularis mucosae, multiple lymphatic invasions were observed (Fig. 1B). Venous invasion was absent (not shown). Within the appendix wall, abscess formation was seen, and atypical epithelial cells were distributed adjacent of the abscess (Fig. 1C). As no malignant cells were observed in the appendiceal mucosa, primary carcinoma of the appendix was unlikely. The serosal membrane was inflammatory but no cancer cells were present. Therefore, there was no possibility of peritoneal dissemination of gastric carcinoma. The adenocarcinoma cells seen in the wall of the appendix were most likely metastatic cancer.
![(A) Macroscopic appearance of the resected appendix vermiformis. Note that the appendix is hyperemic and the serosal surface is white coated. These findings supported the clinical diagnosis of acute appendicitis. (bar = 2 cm). (B) Histology of the resected appendix. Note that multiple submucosal lymphatic channels filled with atypical cell aggregates (arrowheads). These findings suggested metastatic cancer (lymphatic invasion) (Haematoxylin–eosin [HE], ×100). (C) Adjacent to the granulocytes clustering focus (right lower half), many atypical nuclei with conspicuous nucleoli were seen. These figures suggest cancer invasion nearby an abscess (HE, ×400).](https://oupdevcdn.silverchair-staging.com/oup/backfile/Content_public/Journal/jscr/2022/7/10.1093_jscr_rjac322/1/m_rjac322f1.jpeg?Expires=1775988460&Signature=LXm9kNeLvU9r9TtE26Njj3sYT7dbWsJ0tEih8JO-bB9Bzmr970KjksqXKQPICBb412~EWNH0dMn-w-2HgeZGYIpLRQHYh52jCzPOvSQiUWJNSStaFTft6hbyODELb811aMwvGenjAvmHtK6w7OUTfvFuIOGuaPEWzicmgTLbrC53NB-foHfZfzFF0h~Tcr1v3gv~roGWzxzmCAQWaf~JAZIgkeyZaLeoqyzdt0gkMg9f1BILWpmBbughOK1F1z-9bnTuUtfILzCbTUGVc3nwEmjZ3fTp7X9alBRmifg-v0n9C-KgT4qPjD11jVM62YwrSkABnn~9tm3Go0QTdcWIxw__&Key-Pair-Id=APKAIYYTVHKX7JZB5EAA)
(A) Macroscopic appearance of the resected appendix vermiformis. Note that the appendix is hyperemic and the serosal surface is white coated. These findings supported the clinical diagnosis of acute appendicitis. (bar = 2 cm). (B) Histology of the resected appendix. Note that multiple submucosal lymphatic channels filled with atypical cell aggregates (arrowheads). These findings suggested metastatic cancer (lymphatic invasion) (Haematoxylin–eosin [HE], ×100). (C) Adjacent to the granulocytes clustering focus (right lower half), many atypical nuclei with conspicuous nucleoli were seen. These figures suggest cancer invasion nearby an abscess (HE, ×400).
The patient had a history of distal gastrectomy for gastric cancer 2 years before. The pathologic diagnosis was poorly differentiated adenocarcinoma with subserosal invasion (Fig. 2A) and regional lymph nodes metastases (10/35). Lymphatic and venous invasion was extensive. Lymph node metastases were seen along left gastric artery (LGA), right gastric artery and right gastroepiploic artery (RGEA). Lymphatic invasion was evident adjacent to the regional lymph node metastasis (Fig. 2B).
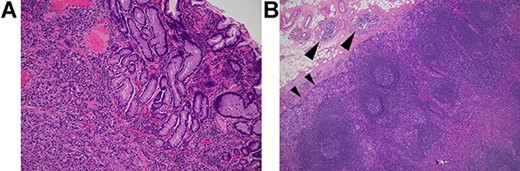
Histology of the primary gastric cancer. (A) Note that highly atypical tumour cells diffusely invade the stomach wall beneath the lamina muscularis mucosae (HE, ×100). (B) Lymph node metastasis (subpyloric node). Note cancer invasion in the peripheral cortex (smaller arrowheads) and afferent lymphoid vessels (larger arrowheads) (HE, ×100). The lymphatic invasion seen here is morphologically similar to those in the appendix vermiformis wall.
These results indicated that the appendiceal metastases likely spread via lymphatic spread. Two months after the appendectomy, multiple peritoneal disseminations developed. The patient deteriorated rapidly and died of intractable ileus and cachexia.
DISCUSSION
Appendiceal malignant neoplasms are uncommon. Among primary tumours, neuroendocrine tumours including carcinoid are most common, and adenocarcinomas, mostly mucinous carcinomas, follow next [1]. Secondary neoplasms of the appendix are further rare. In one series of 139 secondary appendiceal tumours (SATs), stomach is the third common cancer (6.4%) in which the primary sites and SATs are diagnosed simultaneously. However, stomach is the most frequent primary malignancy (57.1%) in which SATs are diagnosed metachronously. In the 139 SATs, 137 cases (98.6%) involved appendix via serosal membrane. Only in two cases (1.4%), the metastasis was restricted in the proper muscle layer or from muscle layer to mucosa [2]. These results indicate that appendiceal metastases usually occur in the advanced stage of cancer via peritoneal dissemination. On the other hand, in the two cases, in which appendiceal metastases were seen only in muscle layer, the cancer cells must have reached to the appendix via haematogenous or lymphatic spread.
In our case, the serosal membrane of the appendix vermiformis was hyperaemic and white-coated, but was not involved with cancer. Therefore, the appendiceal metastasis was not caused via peritoneal dissemination. Extensive lymphatic involvement and lymph node metastases seen in the primary gastric cancer and multiple lymphatic vessels invasion in the appendix suggest that the cancer cells likely metastasized to the appendix via lymphatic spread.
Besides our present case, appendiceal metastases of gastric cancer have been sporadically reported as single case reports [3–12]. None of these were end stage gastric cancer. All these cases were incidentally diagnosed as metastases. Of these, seven cases arose clinically as acute appendicitis [4, 7–12].
The appendiceal metastasis, either via haematogenous or lymphatic spread, seems anatomically improbable since there is no direct connection of lymphatic/venous vessels between the stomach and the appendix. In our case, lymphatic spread was the probable pathway of metastasis because lymphatic channels of both organs were heavily involved with cancer. The lymphatic channels of stomach run along the LGA, the RGEA group, the right gastric artery and the splenic artery. On the other hand, in the appendix vermiformis, the lymph flow from the appendix base drains into ileocolic lymph nodes via anterior and posterior group lymphatic channels.
In a study of regional lymph nodes metastases of 135 cases of gastric cancer, LGA (48.1%) and RGEA (42.2%) are two major lymphatic drainage routes. However, considerable overlap of lymph flow was observed within LGA and RGEA area, and lymph stream was not uni-directional [13]. Skip metastasis in the regional lymph nodes may be explained by the complex network of lymph channels and multi-directional lymph flow in the stomach.
Regional lymph nodes dissection and omentectomy performed during the gastrectomy operation severely disrupt lymph vessel network. Furthermore, reconstruction anastomosis between the duodenum and the remnant stomach/oesophagus creates new aberrant network of lymph channels that never exist in physiological condition. These iatrogenic interventions may facilitate migration of cancer cells to an unpredictable destination. However, the number of reported cases of appendiceal metastasis is very small, which suggests that these unusual lymphatic metastases are stochastically extremely rare.
As the appendix is enriched with lymphoid tissues and numerous lymphatic vessels, cancer cells may be trapped within the appendiceal wall as in lymph node metastasis once cancer cells reach to the appendix.
Appendiceal metastasis itself unlikely invokes clinical symptom. However, when the lymphatic involvement becomes extensive, the lymph flow is obstructed. This may result in lymph-oedema of the appendix, which may make the appendix susceptible to infection and its propagation. This might explain why the appendiceal metastases are often detected clinically as acute appendicitis [4, 7–12].
Although appendiceal metastasis of cancer clinically masquerading acute appendicitis is exceptionally rare, it should be raised as a differential diagnosis in the case with past history of cancer. This case taught us that appendiceal metastasis of cancer may deceptively mimic acute appendicitis clinically and grossly.
CONFLICT OF INTEREST STATEMENT
None to be declared.
FUNDING
No sources of funding to be disclosed.



