-
PDF
- Split View
-
Views
-
Cite
Cite
Muhammad H Mirza, Emeka Nzewi, A rare case of small bowel adenocarcinoma complicating coeliac disease, Journal of Surgical Case Reports, Volume 2022, Issue 6, June 2022, rjac300, https://doi.org/10.1093/jscr/rjac300
Close - Share Icon Share
Abstract
Small bowel adenocarcinoma is an uncommon surgical pathology. Due to non-specific symptoms, most cases present late and pose a challenge to diagnose. We present a case of a small bowel adenocarcinoma in a patient with coeliac disease. A female patient presented to the emergency department with a 3-week history of nausea, anorexia and intermittent bilious vomiting. It was associated with crampy abdominal pain. She was diagnosed with coeliac disease two years ago and commenced on a gluten-free diet. A subsequent computed tomography scan of abdomen and pelvis demonstrated a small bowel stricture with dilated proximal and collapsed distal bowel loops. The stricture was surgically resected followed by primary anastomosis. Histology confirmed adenocarcinoma with nodal metastasis. She received adjuvant chemotherapy and recovered well. In general, small bowel adenocarcinomas are rare and a high index of suspicion is required in patients with predisposing factors e.g. coeliac disease.
INTRODUCTION
Small bowel adenocarcinoma (SBA) accounts for ~40% of all cancers of small bowel [1]. Multiple risk factors and pre-disposing conditions have been described in literature including alcohol and smoking, genetic syndromes e.g. FAP, Lynch Syndrome, Peutz-Jeghers syndrome and inflammatory and immune conditions such as Crohn’s disease and coeliac disease [2]. Coeliac disease (CD) is an autoimmune condition in genetically predisposed individuals, characterized by an immune response triggered after ingestion of gluten. This leads to the formation of autoantibodies causing villous atrophy in the small intestine, with symptoms ranging from malabsorptive syndrome to various extra-intestinal manifestations [3]. The current evidence of correlation between CD and SBA in the literature is predominantly based on case series and population-based studies [4].
The clinical presentation of SBA is non-specific, and a high index of suspicion is required in patients with pre-existing risk factors. As per guidelines, curative surgical resection is the mainstay of treatment, with adjuvant treatment in selective cases. Here, we report a rare case of SBA in a patient with CD.
CASE REPORT
A 63-year-old female presented with a 3-week history of nausea, anorexia, intermittent bilious vomiting, crampy central abdominal pain and weight loss. This was her third presentation to the emergency department since the onset of her symptoms. There was no prior history of altered bowel habits or melena. She previously had a gastroscopy with duodenal biopsies and a colonoscopy for investigation of iron deficiency anaemia 18 months prior to this presentation. Her duodenal biopsies showed villous atrophy and her anti-endomysial antibodies were positive for CD. She was diagnosed with CD and started on a gluten-free diet.
Upon assessment, her abdominal examination revealed tender epigastrium with no palpable mass. A plain abdominal radiograph appeared normal. Subsequently, a computerized tomogram (CT) of her abdomen and pelvis with intravenous contrast was performed, which showed dilated proximal small bowel with a 3-cm stricture and collapsed small bowel distal to the stricture. (Fig. 1). There was lymphadenopathy in the adjacent small bowel mesentery (Fig. 2). A small bowel resection (Proximal Jejunum) was performed through a midline laparotomy. The proximal jejunum was partially obstructed by a 3-cm tumour with multiple lymph nodes visible in the adjacent small bowel mesentery. Resection was done proximally at the duodeno-jejunal (DJ) flexure and distally 15 cm from the tumour in other to perform a satisfactory mesenteric lymphadenectomy (Fig. 3). The third and the fourth parts of the duodenum was partially mobilized and a side-to-side DJ anastomosis was performed.
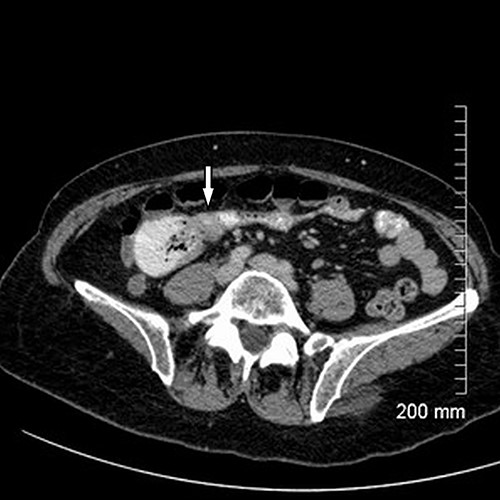
Axial cut of CT abdomen & pelvis demonstrating strictured segment of small bowel (white arrow).
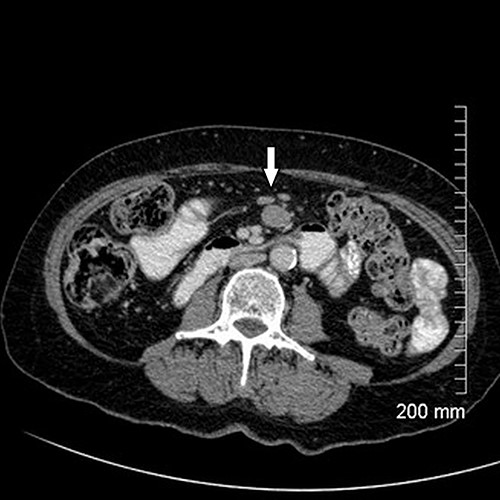
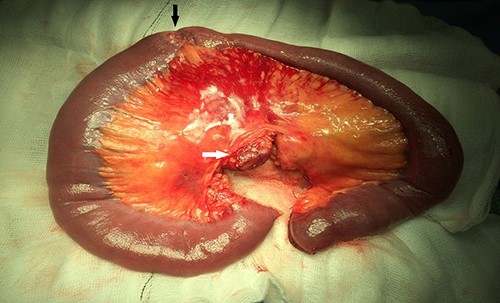
Resected specimen showing the tumour (black arrow) and a large mesenteric lymph node (white arrow).
Histopathological examination revealed a well-differentiated adenocarcinoma of the jejunum, which appears to have risen in a villous lesion invading the submucosa and partially the muscle layer with metastasis to 2 out of 31 lymph nodes. The tumour was staged as pT2 N1 M0 corresponding to Stage IIIa. The patient had an uneventful recovery and discharged home. Following surgery, she received adjuvant chemotherapy comprising 12 cycles of FOLFOX (Folinic acid, 5-Fluorouracil and Oxaliplatin), which was well tolerated.
DISCUSSION
Clinical presentation of SBA is usually non-specific. A large case series from the department of oncology, Mayo Clinic [5] analysed records of 491 patients with SBA ranging over 35 years. They reported abdominal pain as the most frequent symptom, followed by nausea and vomiting, anaemia and overt gastrointestinal bleeding. In our case, nausea and bilious vomiting were more pronounced due to proximal location of the tumour. In patients with more distal lesions, crampy abdominal pain is usually more prominent.
The pathogenesis of SBA is similar to that of large bowel adenocarcinoma in that former is considered to arise from a pre-existing adenoma [6]. However, SBA have a different genomic profile compared with colorectal and gastric adenocarcinomas, including variations in the frequency and types of alterations of adenomatosis polyposis coli (APC), human epidermal growth factor receptor 2 (HER2) and other genes [7, 8].
Presence of aforementioned symptoms in a patient of CD should warrant early investigations to out rule a malignant process. A current review from Michigan State University has described a combination of laboratory tests, radiological investigations and endoscopic evaluation for diagnosis of small bowel neoplasms [9]. Routine laboratory workup includes full blood count, which can reveal previously undiagnosed anaemia secondary to occult bleeding from tumour. In contrast to NETs (Neuroendocrine Tumours), no specific serological biomarkers for SBA have been described. The role of tumour markers such as CEA and CA 19–9 is significant in post-treatment surveillance.
Prior to the formulation of guidelines for SBA treatment, its management strategy was similar to that of CRC. In 2018, a French intergroup first formulated a set of guidelines for the treatment of SBA [10]. In September 2019, NCCN (National Comprehensive Cancer Network) Clinical Practice Guidelines in Oncology for Small Bowel Adenocarcinoma were published [11]. Both guidelines recommend surgical resection as the curative treatment, after consideration of the disease and patient’s factors. For adenocarcinoma located in jejunum, as in our patient’s case, a segmental resection with lymph node dissection is recommended [10, 11]. Adjuvant therapy such as chemotherapy should be employed in carefully selected cases in a multi-disciplinary setting.
CONCLUSION
CD is a recognized predisposing clinical condition for development of SBA. Early diagnosis of SBA is the key. A high index of suspicion is required for underlying malignancy in patients with CD who have poor compliance with gluten-free diet, refractory CD and other co-existing risk factors. Curative surgical resection, with adjuvant treatment in selected cases is the standard management for SBA. Further research is essential to establish the role of endoscopic surveillance and gluten free diet in the prevention of SBA in patients with CD.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
- anorexia nervosa
- computed tomography
- small intestinal stricture
- celiac disease
- adenocarcinoma
- adjuvant chemotherapy
- anastomosis, surgical
- constriction, pathologic
- disease susceptibility
- emergency service, hospital
- intestines
- nausea
- surgical pathology
- abdomen
- histology
- pelvis
- small intestine adenocarcinoma
- lymph node metastasis
- loss of appetite
- gluten free diet
- vomiting bile
- abdominal cramps



