-
PDF
- Split View
-
Views
-
Cite
Cite
Yusuke Nishina, Hiroyuki Ohta, Yoshitaka Terada, Hiroya Akabori, Naomi Kitamura, Nozomi Nagai, Eiji Mekata, Successful treatment of rectal cancer with pelvic abscess using transrectal drainage followed by laparoscopic radical resection: a case report, Journal of Surgical Case Reports, Volume 2022, Issue 6, June 2022, rjac284, https://doi.org/10.1093/jscr/rjac284
Close - Share Icon Share
Abstract
The incidence of rectal cancer with a pelvic abscess is rare; hence, treatment strategies are difficult because both malignant and infectious inflammation need to be addressed. Here, we report the case of a 53-year-old man diagnosed with rectal cancer accompanied by a pelvic abscess. We performed transrectal drainage of the abscess, and a transanal rectal drainage tube was inserted into the abscess cavity. His symptoms rapidly improved, and computed tomography showed that the pelvic abscess had disappeared. Six weeks after drainage, radical laparoscopic Hartmann’s procedure with resection of the rectal cancer and incision drainage scar was performed. After adjuvant chemotherapy, laparoscopic stoma closure was performed a year after the operation. The patient showed no evidence of cancer recurrence 1.5 years after radical surgery. Transrectal drainage followed by laparoscopic radical resection can be a less invasive and effective treatment for rectal cancer accompanied by a pelvic abscess.
INTRODUCTION
Rectal cancer is rarely accompanied by perirectal abscesses. A concomitant pelvic abscess due to tumor perforation could be a high-risk factor for local recurrence in patients with rectal cancer [1, 2]. Infection control and radical resection surgery are often difficult to perform. We report the case of a patient who underwent successful laparoscopic resection of rectal cancer after transrectal drainage of an accompanying pelvic abscess.
CASE REPORT
A 53-year-old Japanese man was admitted to the hospital with a chief complaint of buttock pain that lasted for 5 months and a recent weight loss of 8 kg, from 68 kg to 60 kg. He had no remarkable medical history. Digital rectal examination could not be performed because of severe pain. Laboratory findings revealed severe inflammation, indicated by an increased white blood cell count (16 790 cells/μL) and C-reactive protein (12.15 mg/dL). He was also undernourished with an albumin level of 2.7 g/dL. Tumor marker levels of carcinoembryonic antigen and cancer antigen 19–9 were within the normal ranges. Pelvic computed tomography (CT) showed contrast-enhanced thickening of the upper rectal wall and perirectal abscess (Fig. 1). Swollen lymph nodes were observed near the mesentery, but distant metastases were not detected. Rectal cancer accompanied by a pelvic abscess was then suspected. For the patient’s relief, sigmoid colonoscopy was performed under lumbar spinal anesthesia, which revealed a semicircular type 2 tumor in the upper rectum (Fig. 2a), and exclusion from outside the rectal wall on the anal side of the tumor at 7 cm from the anal verge (Fig. 2b). The colonoscope could not pass through the oral side of the tumor.
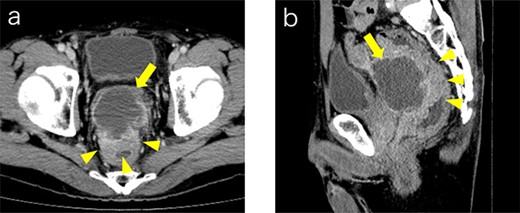
Contrast medium-enhanced CT upon admission in axial (a) and sagittal (b) sections. Irregular thickening of the rectal wall (arrowheads) and an accompanying pelvic abscess (arrows) are observed. No evident distant metastases are observed.
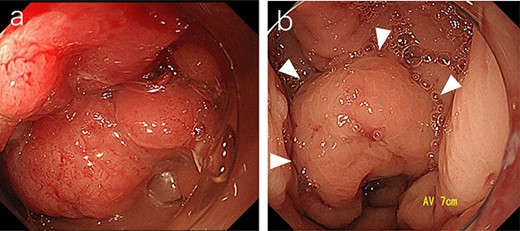
Sigmoid colonoscopy revealed a circular tumor in the upper rectum (a). Extramural compression due to an abscess is observed on the anal canal side of the tumor (arrowheads); however, the penetrated portion could not be detected (b).
Subsequently, we performed transrectal drainage of the abscess in the lithotomy position without endoscopy. After the incision of the excluded rectal mucosa at 7 cm from the anal verge by the electric scalpel and penetration of the abscess wall by the Kelly clamp, a massive purulent discharge was observed. Next, a transanal rectal drainage tube (MIT drain®, effective length 12 cm; Create Medic Co., Ltd, Yokohama, Japan) was inserted into the abscess cavity without the use of a guide wire, and saline irrigation was performed. After transrectal drainage, the patient’s pain and inflammatory laboratory findings improved rapidly. Pelvic CT showed shrinkage of the cavity 3 days after the drainage (Fig. 3). Bacterial culture test and cytology of the drainage contents revealed negative growth and no malignancy, respectively. The biopsy results showed moderately differentiated tubular adenocarcinoma. The inserted tube was removed on the seventh day after the drainage, and the patient was discharged on the eighth day. In the outpatient department, total colonoscopy revealed that the exclusion from outside the rectal wall on the anal side of the tumor diminished, and the incised mucosa healed as a scar. Ink sticks were marked near the scar on behalf of the combined resection of the tumor. Pelvic CT revealed that the pelvic abscess had disappeared 1 month after drainage (Fig. 4).
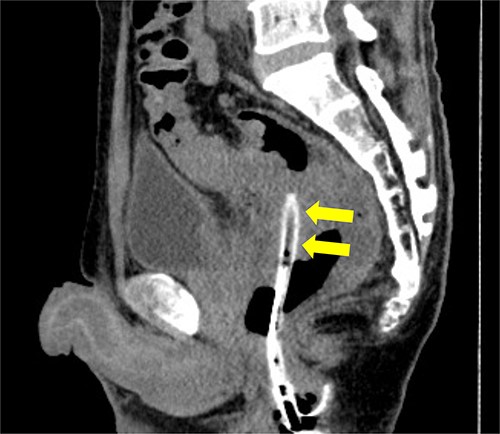
Pelvic CT performed 3 days after drainage in the sagittal section. Abscess cavity remarkably shrunk (arrowheads).
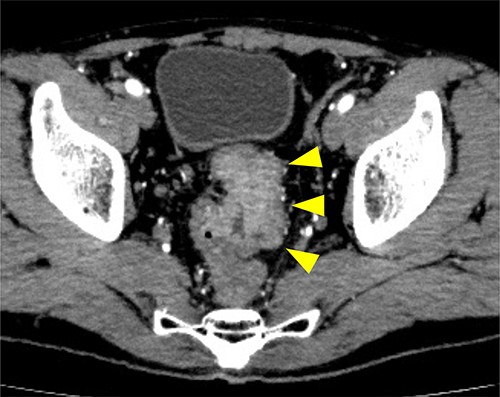
Preoperative CT examination one month after drainage. Thickening of the rectal wall is demonstrated (arrowheads); however, the pelvic abscess completely disappeared.
The patient’s nutritional status significantly improved 6 weeks after drainage; therefore, radical surgery for rectal cancer was performed under general anesthesia. During laparoscopic surgery, the serosa on the tumor was twitched, and the adhesion between the anterior wall of the rectum on the anal side of the tumor and the peritoneal reflection became rigid. No peritoneal dissemination was observed. Hartmann’s procedure without primary anastomosis was performed considering the possibility of rectal cancer recurrence. The resected specimen containing the transrectal drainage route is shown in Fig. 5. The pathology report revealed a moderately differentiated adenocarcinoma in the upper rectum, pT3 pN0 M0 pStage IIa (TNM classification 8th edition). The postoperative clinical course was uneventful, and the patient was discharged on the 15th postoperative day. Postoperative adjuvant chemotherapy of CAPEOX was administered for 6 months, and laparoscopic stoma closure was performed a year after the operation, following the patient’s request. He had no evidence of cancer recurrence 1.5 years after radical surgery.
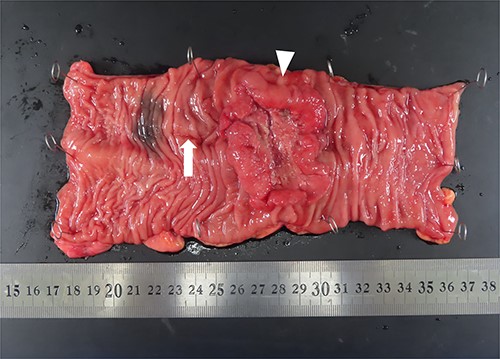
Macroscopic findings of the resected specimen. A circumferential tumor (arrowhead) and mucosal scar upon presenting the drainage route (arrow) are located in the rectum.
DISCUSSION
Rectal cancer is rarely diagnosed as a pelvic abscess due to simultaneous tumor perforation, leading to postoperative local recurrence. Infection control is crucial for preventing sepsis in patients with rectal cancer accompanied by abscesses.
The drainage route for the pelvic abscess was considered in this case. Percutaneous drainage through the perineum can cause dissemination of tumor cells, thereby encouraging local recurrence. Moreover, a one-stage transabdominal drainage of the abscess and radical resection of the tumor also pose a risk for peritoneal dissemination. Conversely, transrectal drainage prevents dissemination within the shortest distance to the abscess and uses a less invasive procedure. However, the transrectal drainage route, where tumor cells can be disseminated, should be resected in presence of rectal cancer. Therefore, a drainage incision should be performed on the anal canal side near the tumor, especially before anal-preserving radical surgery.
In our case, the pelvic abscess was large enough that the drainage site was identifiable because of the distinction of the rectal mucosa. However, small abscesses may be difficult to identify. Attempts to precisely detect pelvic abscesses during transrectal drainage, such as echo-guided, magnetic resonance imaging-guided, and endoscopic drainage, have been reported [3–5]. Based on these reports, these modalities were indicated for benign lesions, such as appendicitis, Crohn’s disease, postoperative infection and trauma. To the best of our knowledge, there is only one report of transrectal drainage for pelvic abscess due to rectal cancer conducted in Japan [6], but none in the Western literature.
From the point of view of the rectal tumor and abscess location, transrectal drainage may be indicated when the tumors and pelvic abscesses are at some distance from the anal verge, and anal-preserving surgery can be expected. Otherwise, percutaneous drainage may be useful when the tumors accompanied by abscesses are close to the anal verge and require abdominoperineal resection.
In conclusion, transrectal drainage prior to anal-preserving radical surgery may be a less invasive and effective treatment for pelvic abscesses due to rectal cancer. Surgical resection of the tumor and drainage route can be a radical cure for rectal cancer with a pelvic abscess.
CONFLICT OF INTEREST STATEMENT
None declared.
CONSENT FOR PUBLICATION
Written informed consent for publication of this case report was obtained from the patient.
ACKNOWLEDGEMENTS
The authors would like to thank Editage (www.editage.com) for English language editing.



