-
PDF
- Split View
-
Views
-
Cite
Cite
Jo Ohta, Yuji Kadoi, Masahiko Tosaka, Shigeru Saito, Pseudo-intestinal obstruction after transsphenoidal surgery for craniopharyngioma, Journal of Surgical Case Reports, Volume 2022, Issue 5, May 2022, rjac254, https://doi.org/10.1093/jscr/rjac254
Close - Share Icon Share
Abstract
Craniopharyngioma surgery is frequently associated with the occurrence of central diabetes insipidus, and oral rehydration therapy is reliable for postoperative management if the patient’s thirst is normal. A 61-year-old Japanese male patient underwent extended endoscopic transsphenoidal surgery for craniopharyngioma. He was undergoing acute treatment for postoperative central diabetes insipidus and hypopituitarism in the intensive care unit. Two days after the surgery, he started to vomit occasionally, despite receiving oral rehydration therapy for central diabetes insipidus. Despite increasing the dose of parenteral hydrocortisone, the periodic vomiting persisted during fasting periods and progressed to aspiration pneumonia and severe sepsis. Abdominal computed tomography was performed to identify the cause of persistent vomiting and revealed the presence of a pseudo-intestinal obstruction extending from the small to large intestine. When oral rehydration therapy for central diabetes insipidus is accompanied by vomiting symptoms suggestive of hypopituitarism, a holistic evaluation of the gastrointestinal system is advisable.
INTRODUCTION
Craniopharyngioma carries a high risk of endocrinological complications after surgery [1]. In particular, central diabetes insipidus (CDI) and hypopituitarism are frequent and often concomitant. CDI is characterized by polyuria, thirst and increased plasma osmolarity, while hypopituitarism symptoms are nonspecific [2] and include nausea and vomiting, which puts it at risk of being confused with other diseases. Here, we report a case of repeated periodic vomiting after craniopharyngioma surgery, in which the direct cause was not hypopituitarism but pseudo-intestinal obstruction. In the present case, oral rehydration therapy for CDI was inhibited by pseudo-intestinal obstruction, suggesting the importance of gastrointestinal monitoring.
CASE REPORT
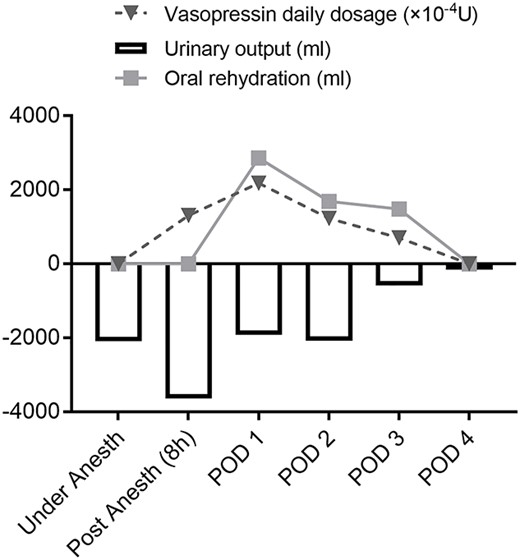
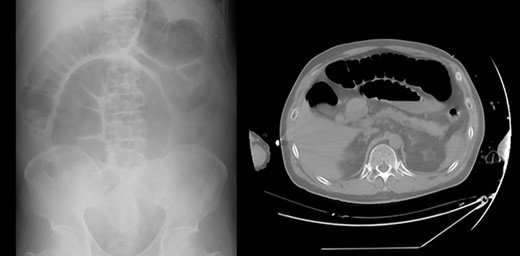
A 61-year-old man complained of progressive vision loss on the right side and visual field loss on the left side of the ear. Head magnetic resonance imaging examination showed a 26 × 18 × 24 mm pituitary suprasellar cystic tumor with enhancement on T1-weighted images after gadolinium injection. The tumor contained septal-like structures consistent with craniopharyngioma. He had a history of glaucoma and chronic atrial fibrillation and was undergoing medical treatment. The pituitary gland was within the range of normal endocrine function before the surgery. Consequently, he underwent extended endoscopic transsphenoidal surgery. Pituitary function was lost and the pituitary stalk was amputated for complete tumor removal. On the day of surgery, he was diagnosed with CDI, showing polyuria (3640 ml) in 8 hours. He was started on intravenous vasopressin with an index serum sodium level of 140–145 (mEq/l) and oral rehydration therapy based on thirst (Fig. 1; Table 1). For hypopituitarism, glucocorticoid coverage was initially planned as 100 mg of intraoperative and postoperative hydrocortisone on the day of surgery, followed by intravenous administration of 50 mg for 2 days and 30 mg for 1 day. On the second day, he showed occasional episodes of vomiting, a symptom of hypopituitarism. As a result, parenteral hydrocortisone was increased to 80 mg that day and 50 mg on the fourth day. However, periodic vomiting continued, and despite the fasting period, the patient developed aspiration pneumonia on the third day, which progressed to severe sepsis on the fourth day (Table 1). Abdominal radiography and computed tomography revealed pseudo-intestinal obstruction as a direct cause of vomiting (Fig. 2). During multidisciplinary treatment for severe sepsis, he defecated on postoperative day 14, and abdominal radiography showed improvement of pseudo-intestinal obstruction. He was discharged on Day 79 with a prescription for oral glucocorticoid and intranasal desmopressin.
| . | Baseline . | POD 1 . | POD 2 . | POD 3 . | POD 4 . |
|---|---|---|---|---|---|
| Plasma osmolality (mosm/kg) | 293.6 | 313.7 | 283.8 | 287.4 | 289.6 |
| pH | - | 7.481 | 7.471 | 7.474 | 7.247 |
| BE (mmol/l) | - | 1.8 | 0.1 | −1.2 | −6.9 |
| HCO−3 (mmol/l) | - | 25 | 23.3 | 20.9 | 19.1 |
| Serum sodium (mEq/l) | 141 | 152 | 137 | 137 | 136 |
| Serum potassium (mEq/l) | 4.2 | 3.7 | 2.9 | 3.0 | 3.1 |
| Serum chloride (mEq/l) | 105 | 117 | 113 | 102 | 101 |
| P/F ratio | - | 443.8 | 300.0 | 67.7 | 77.7 |
| T-bil (mg/dl) | 1.3 | 1.2 | 1.8 | 1.9 | 3.0 |
| AST (IU/l) | 18 | 24 | 25 | 21 | 18 |
| ALT (IU/l) | 14 | 23 | 25 | 24 | 13 |
| BUN (mg/dl) | 16 | 10 | 10 | 16 | 30 |
| Cr (mg/dl) | 0.9 | 0.85 | 0.76 | 1.19 | 3.07 |
| eGFR (m/min/m2) | 66.9 | 71.2 | 80.5 | 49.3 | 17.5 |
| . | Baseline . | POD 1 . | POD 2 . | POD 3 . | POD 4 . |
|---|---|---|---|---|---|
| Plasma osmolality (mosm/kg) | 293.6 | 313.7 | 283.8 | 287.4 | 289.6 |
| pH | - | 7.481 | 7.471 | 7.474 | 7.247 |
| BE (mmol/l) | - | 1.8 | 0.1 | −1.2 | −6.9 |
| HCO−3 (mmol/l) | - | 25 | 23.3 | 20.9 | 19.1 |
| Serum sodium (mEq/l) | 141 | 152 | 137 | 137 | 136 |
| Serum potassium (mEq/l) | 4.2 | 3.7 | 2.9 | 3.0 | 3.1 |
| Serum chloride (mEq/l) | 105 | 117 | 113 | 102 | 101 |
| P/F ratio | - | 443.8 | 300.0 | 67.7 | 77.7 |
| T-bil (mg/dl) | 1.3 | 1.2 | 1.8 | 1.9 | 3.0 |
| AST (IU/l) | 18 | 24 | 25 | 21 | 18 |
| ALT (IU/l) | 14 | 23 | 25 | 24 | 13 |
| BUN (mg/dl) | 16 | 10 | 10 | 16 | 30 |
| Cr (mg/dl) | 0.9 | 0.85 | 0.76 | 1.19 | 3.07 |
| eGFR (m/min/m2) | 66.9 | 71.2 | 80.5 | 49.3 | 17.5 |
POD, postoperative day; BE, base excess; P/F, PaO2/FiO2; T-bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; eGFR, estimated glomerular filtration rate.
| . | Baseline . | POD 1 . | POD 2 . | POD 3 . | POD 4 . |
|---|---|---|---|---|---|
| Plasma osmolality (mosm/kg) | 293.6 | 313.7 | 283.8 | 287.4 | 289.6 |
| pH | - | 7.481 | 7.471 | 7.474 | 7.247 |
| BE (mmol/l) | - | 1.8 | 0.1 | −1.2 | −6.9 |
| HCO−3 (mmol/l) | - | 25 | 23.3 | 20.9 | 19.1 |
| Serum sodium (mEq/l) | 141 | 152 | 137 | 137 | 136 |
| Serum potassium (mEq/l) | 4.2 | 3.7 | 2.9 | 3.0 | 3.1 |
| Serum chloride (mEq/l) | 105 | 117 | 113 | 102 | 101 |
| P/F ratio | - | 443.8 | 300.0 | 67.7 | 77.7 |
| T-bil (mg/dl) | 1.3 | 1.2 | 1.8 | 1.9 | 3.0 |
| AST (IU/l) | 18 | 24 | 25 | 21 | 18 |
| ALT (IU/l) | 14 | 23 | 25 | 24 | 13 |
| BUN (mg/dl) | 16 | 10 | 10 | 16 | 30 |
| Cr (mg/dl) | 0.9 | 0.85 | 0.76 | 1.19 | 3.07 |
| eGFR (m/min/m2) | 66.9 | 71.2 | 80.5 | 49.3 | 17.5 |
| . | Baseline . | POD 1 . | POD 2 . | POD 3 . | POD 4 . |
|---|---|---|---|---|---|
| Plasma osmolality (mosm/kg) | 293.6 | 313.7 | 283.8 | 287.4 | 289.6 |
| pH | - | 7.481 | 7.471 | 7.474 | 7.247 |
| BE (mmol/l) | - | 1.8 | 0.1 | −1.2 | −6.9 |
| HCO−3 (mmol/l) | - | 25 | 23.3 | 20.9 | 19.1 |
| Serum sodium (mEq/l) | 141 | 152 | 137 | 137 | 136 |
| Serum potassium (mEq/l) | 4.2 | 3.7 | 2.9 | 3.0 | 3.1 |
| Serum chloride (mEq/l) | 105 | 117 | 113 | 102 | 101 |
| P/F ratio | - | 443.8 | 300.0 | 67.7 | 77.7 |
| T-bil (mg/dl) | 1.3 | 1.2 | 1.8 | 1.9 | 3.0 |
| AST (IU/l) | 18 | 24 | 25 | 21 | 18 |
| ALT (IU/l) | 14 | 23 | 25 | 24 | 13 |
| BUN (mg/dl) | 16 | 10 | 10 | 16 | 30 |
| Cr (mg/dl) | 0.9 | 0.85 | 0.76 | 1.19 | 3.07 |
| eGFR (m/min/m2) | 66.9 | 71.2 | 80.5 | 49.3 | 17.5 |
POD, postoperative day; BE, base excess; P/F, PaO2/FiO2; T-bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; eGFR, estimated glomerular filtration rate.
DISCUSSION
We report a rare case of craniopharyngioma presenting with postoperative periodic vomiting due to pseudo-intestinal obstruction during oral rehydration therapy for CDI.
Water metabolism disorders associated with craniopharyngioma surgery range from diabetes insipidus to inappropriate antidiuretic hormone secretion syndrome and cerebral salt-wasting syndrome [2, 3]. CDI, which is most likely to occur, requires continuous monitoring of serum sodium levels and urine output, and replacement of fluid loss. Vasopressin or its analog continue to be given as a basal requirement to avoid water intoxication and hyponatremia until the CDI is determined to be permanent. It is important to assess whether the thirst mechanism is preserved, and if the patient’s thirst is intact, oral rehydration is recommended as a convenient way to alleviate increased serum sodium concentrations and plasma osmolality [2, 3].
Pseudo-intestinal obstruction is a relatively rare morbid condition [4, 5]. Although the exact mechanism of acute onset has not been elucidated, there is strong support for increased sympathetic tone and decreased parasympathetic tone via several pathways due to multifactorial etiology [4]. The prognosis is poor in cases of ischemia or perforation, but most cases improve within several days with conservative treatment [5]. In the present case, oral rehydration for CDI decreased during the fasting period due to periodic vomiting, while vasopressin requirements decreased with normalization of sodium levels. Since the patient developed permanent CDI and hypopituitarism after surgery, we believe that this is not due to a transient improvement in CDI, but rather to an enhanced masking effect of cortisol deficiency on CDI [2–6]. There have been several reports of relative adrenal insufficiency in critically ill patients presenting with pseudo-intestinal obstruction [7, 8]. Hypopituitarism-related gastrointestinal symptoms have also been reported to be associated with decreased serum sodium and cortisol levels [9]. Thus, while pseudo-intestinal obstruction was the direct cause of the periodic vomiting here, we believe that an association with hypopituitarism cannot be excluded. All craniopharyngioma surgeries carry similar risks, suggesting that careful monitoring of the gastrointestinal system should accompany oral rehydration in cases of concomitant CDI.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
REFERENCES
- hydrocortisone
- aspiration pneumonia
- central diabetes insipidus
- craniopharyngioma
- endoscopy
- gastrointestinal system
- fasting
- hypopituitarism
- intensive care unit
- intestine, large
- intestines
- postoperative care
- surgical procedures, operative
- thirst
- vomiting
- persistence
- sepsis, severe
- rehydration therapy
- abdominal ct
- cyclical vomiting syndrome
- japanese



