-
PDF
- Split View
-
Views
-
Cite
Cite
Neha Jadhav, Lawrence Sivakumar, Sayed Samed Talibi, Pearl Momoh, Fahid Rasul, Rahim Hussain, Amjad Shad, Lumbar discal cyst and post-operative discal pseudocyst: a case series, Journal of Surgical Case Reports, Volume 2022, Issue 5, May 2022, rjac239, https://doi.org/10.1093/jscr/rjac239
Close - Share Icon Share
Abstract
Four cases of lumbar cyst (2 discal cysts and 2 post-operative discal pseudocysts) who presented predominantly with features of sciatica without any motor, sensory or sphincteric disturbances. The patients were treated conservatively, and the management was aimed to avert any untoward surgical intervention taking into consideration patient safety and care. Two had previous lumbar decompressive discectomy. During the mean follow-up period of 13 months, there was progressive recovery of symptoms in all our 4 patients. All our patients were successfully managed by conservative approach. An intervertebral disc cyst should be considered in young patients in the differential diagnosis of any extradural intraspinal mass ventral to the thecal sac, notwithstanding its rarity. Alongside, conservative management can be offered as first line of management with appropriate patient selection that is absence of any motor/sensory/sphincteric disturbances. Facetal micro-instability could be one of the aetiologies of this pathology which necessitates further study.
INTRODUCTION
Post-operative discal pseudocyst (PDP) is a rare condition that develops after surgery for lumbar disc herniation. Post-PDP as a pathological condition was only reported over a decade ago and characterized as a cystic lesion that is attached to the site of surgery at the disc that underwent discectomy [1, 2]. The pathogenesis of post-PDP remains unclear [3–5]. Discal cyst (DC) unlike another cystic lesion can be typically found at the intervertebral disc. The two principal strategies for the treatment of post-PDP are conservative treatment and surgical cystectomy, both are reportedly effective and the indications for the treatments remain controversial. Post-PDP and DC exhibit similarities in both histological and epidemiological characteristics, which indicates the same likely pathological origin of post-PDP and DC [6]. The iatrogenic annular injury during discectomy might accelerate the pathological progression of DC. For patients with mild to moderate symptoms, conservative treatment can lead to great improvement, even inducing spontaneous regression. Surgical cystectomy is necessary in patients with neurological deficits and where conservative treatment is ineffective [7].
In this article, we share our experience of conservatively managed cases of both the lumbar DC and post-PDP and their satisfaction at interval follow-up.
MATERIALS AND METHODS
Four patients who were treated in the Neurosurgery Department of the authors between 2019 and 2021 comprised the study material. The patient provided written informed consent, although their data were anonymized, and the clinical tests were conducted according to the principles of declaration of Helsinki. Local hospital trust ethical policies were adhered to.
RESULTS
The clinical profile of the patients is summarized in Tables 1–3. Three patients were males, one female and their ages ranged from 25 to 46 years (average 33.5 years) (Table 4). The median follow-up period was 12-months from diagnosis (Table 5).
Radiological analysis
The diagnosis was reached keeping in mind the radiological and contrast scan was taken in follow-up period (Figs 1, 2a, b,3a, b and4a, b). On MRI, it was noted that the cyst was well-defined, smooth regular margins with T1 hypointense and T2 hyperintense and an evident communicating stalk with the disc while in post-gadolinium scan showed cyst wall enhancement. As discography is no longer required for reaching a diagnosis, it was not done in any of the patients.
| Sex | |
| Male | 1 |
| Female | 1 |
| Mean Age | 27 |
| Levels involved | |
| L4-L5 | 1 |
| L5-S1 | 1 |
| Number of levels of discectomy | |
| 1 | 1 (L5-S1) |
| 2 | 1 (L4–5, L5-S1) |
| Sex | |
| Male | 1 |
| Female | 1 |
| Mean Age | 27 |
| Levels involved | |
| L4-L5 | 1 |
| L5-S1 | 1 |
| Number of levels of discectomy | |
| 1 | 1 (L5-S1) |
| 2 | 1 (L4–5, L5-S1) |
| Sex | |
| Male | 1 |
| Female | 1 |
| Mean Age | 27 |
| Levels involved | |
| L4-L5 | 1 |
| L5-S1 | 1 |
| Number of levels of discectomy | |
| 1 | 1 (L5-S1) |
| 2 | 1 (L4–5, L5-S1) |
| Sex | |
| Male | 1 |
| Female | 1 |
| Mean Age | 27 |
| Levels involved | |
| L4-L5 | 1 |
| L5-S1 | 1 |
| Number of levels of discectomy | |
| 1 | 1 (L5-S1) |
| 2 | 1 (L4–5, L5-S1) |
| Sex | |
| Male | 2 |
| Female | - |
| Mean Age | 40 |
| Levels involved | |
| L4-L5 | - |
| L5-S1 | 2 |
| Sex | |
| Male | 2 |
| Female | - |
| Mean Age | 40 |
| Levels involved | |
| L4-L5 | - |
| L5-S1 | 2 |
| Sex | |
| Male | 2 |
| Female | - |
| Mean Age | 40 |
| Levels involved | |
| L4-L5 | - |
| L5-S1 | 2 |
| Sex | |
| Male | 2 |
| Female | - |
| Mean Age | 40 |
| Levels involved | |
| L4-L5 | - |
| L5-S1 | 2 |
| Presenting complaints . | Number of patients . |
|---|---|
| Back pain | 4 |
| Leg pain | |
| Unilateral | 3 |
| Bilateral | 1 |
| Tingling paraesthesia | 4 |
| Weakness in one or more muscle groups | - |
| Presenting complaints . | Number of patients . |
|---|---|
| Back pain | 4 |
| Leg pain | |
| Unilateral | 3 |
| Bilateral | 1 |
| Tingling paraesthesia | 4 |
| Weakness in one or more muscle groups | - |
| Presenting complaints . | Number of patients . |
|---|---|
| Back pain | 4 |
| Leg pain | |
| Unilateral | 3 |
| Bilateral | 1 |
| Tingling paraesthesia | 4 |
| Weakness in one or more muscle groups | - |
| Presenting complaints . | Number of patients . |
|---|---|
| Back pain | 4 |
| Leg pain | |
| Unilateral | 3 |
| Bilateral | 1 |
| Tingling paraesthesia | 4 |
| Weakness in one or more muscle groups | - |
| Patient . | Radicular Pain . | Side . | Paraesthesia . | Sensory findings . | Sphincteric disturbances . | Motor findings . |
|---|---|---|---|---|---|---|
| 1 | + | L > R | + | − | − | − |
| 2 | + | L | + | − | − | − |
| 3 | + | L | + | − | − | − |
| 4 | + | L | + | − | − | − |
| Patient . | Radicular Pain . | Side . | Paraesthesia . | Sensory findings . | Sphincteric disturbances . | Motor findings . |
|---|---|---|---|---|---|---|
| 1 | + | L > R | + | − | − | − |
| 2 | + | L | + | − | − | − |
| 3 | + | L | + | − | − | − |
| 4 | + | L | + | − | − | − |
[+] = sign/symptom present
[−] = sign/symptom absent
| Patient . | Radicular Pain . | Side . | Paraesthesia . | Sensory findings . | Sphincteric disturbances . | Motor findings . |
|---|---|---|---|---|---|---|
| 1 | + | L > R | + | − | − | − |
| 2 | + | L | + | − | − | − |
| 3 | + | L | + | − | − | − |
| 4 | + | L | + | − | − | − |
| Patient . | Radicular Pain . | Side . | Paraesthesia . | Sensory findings . | Sphincteric disturbances . | Motor findings . |
|---|---|---|---|---|---|---|
| 1 | + | L > R | + | − | − | − |
| 2 | + | L | + | − | − | − |
| 3 | + | L | + | − | − | − |
| 4 | + | L | + | − | − | − |
[+] = sign/symptom present
[−] = sign/symptom absent
| Patient . | Radicular Pain . | Paraesthesia . | Sensory findings . | Sphincteric disturbances . | Motor findings . |
|---|---|---|---|---|---|
| 1 | - | - | - | - | - |
| 2 | - | - | - | - | - |
| 3 | - | - | - | - | - |
| 4 | - | - | - | - | - |
| Patient . | Radicular Pain . | Paraesthesia . | Sensory findings . | Sphincteric disturbances . | Motor findings . |
|---|---|---|---|---|---|
| 1 | - | - | - | - | - |
| 2 | - | - | - | - | - |
| 3 | - | - | - | - | - |
| 4 | - | - | - | - | - |
[+] = sign/symptom present
[−] = sign/symptom absent
| Patient . | Radicular Pain . | Paraesthesia . | Sensory findings . | Sphincteric disturbances . | Motor findings . |
|---|---|---|---|---|---|
| 1 | - | - | - | - | - |
| 2 | - | - | - | - | - |
| 3 | - | - | - | - | - |
| 4 | - | - | - | - | - |
| Patient . | Radicular Pain . | Paraesthesia . | Sensory findings . | Sphincteric disturbances . | Motor findings . |
|---|---|---|---|---|---|
| 1 | - | - | - | - | - |
| 2 | - | - | - | - | - |
| 3 | - | - | - | - | - |
| 4 | - | - | - | - | - |
[+] = sign/symptom present
[−] = sign/symptom absent
Management
All the patients were managed conservatively which included analgesia, neuropathic agents and when required, physiotherapy and certain precautions such as avoiding heavy lifting, but no strict bed rest was advised. Patients were educated about the red flag signs such as sensory or motor weakness, sphincteric disturbances as well pain out of proportion not responding to medical therapy. The patients were asked to report to their nearest hospital if they noticed any of the above-mentioned red flag signs. While surgery was not the primary option given to the patients, it was available if at any point, they failed with expectant conservative management. At 12-months, the patients remained concordant with their medical therapy and no surgery was carried out.
DISCUSSION
Post-PDP and DC exhibit similarities in both histological and epidemiological characteristics, which indicates the same pathological origin of post-PDP and DC [6].
Lumbar spine pathologies including herniated nucleus pulposus, perineural cyst, extradural arachnoid cyst, synovial cyst of the facet joint and ganglion cyst have clinical features such as those of DC [8, 9]. The pathogenesis differs although often results in symptoms and signs indistinguishable from disc herniation, common symptoms including lower back pain and radiculopathy [10, 11].
The management of these intradiscal cystic lesions is variable ranging from monitoring, medical therapies, CT-guided aspirations, epidural injections, surgical resection ± discectomy, and in some reported cases, the cysts were noted to resolve spontaneously [2, 9–14].
DCs are often well-defined homogenous cyst located in the ventrolateral extradural space, which displaces the dural sac dorsomedially and communicates with the intervertebral disc [15]. Intradiscal cystic lesions can be often located extradurally with a distinct communication with the corresponding intervertebral discs, whereas DCs differ such that they are a rarity, preponderance for the lumbar spine, younger age groups are primary affected and by predominantly affecting male than female patients by a ratio of 9:1 [3, 9–11, 15]. In this case, three are male and one case female, with the average age of 33.5 years (25–46 years), consistent with what has been described in the literature.
There is a distinct lack of large-scale studies, and the aetiology of DCs remains elusive, yet two hypotheses do exist. One hypothesis is that an acute, significant mechanical stress event/injury results in a more acute formation of disc herniation which in turn results in acute degeneration of the disc itself and leading to fluid collection and an inflammatory reaction and subsequent pseudo-membrane formation—the ‘rapid’ nature of this development leads to symptoms developing sooner compared with degenerative disc herniation usually seen in the general population at an older age, which develops over years of progressive and sustained pressure on the disc/vertebrae [2, 9, 12–14]. It is not clearly understood whether an underlying defect in the annulus fibrosus predisposes to DCs in this fashion, i.e. a genetic component or whether the significant nature of the acute high-force mechanical stress event/injury more readily causes disruption of the annulus fibrosus (likely in the posterior intervertebral disc) leading to nucleus fluid extrusion propagating the inflammatory response and the subsequent pseudo-membrane formation—becoming a DC [2, 9, 12–14].
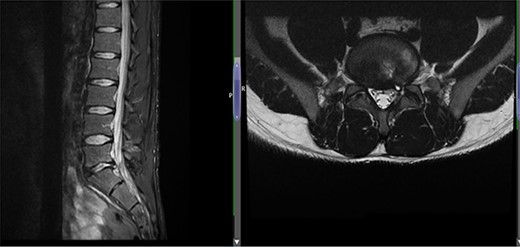
Case 1 L5-S1 level: there is disc desiccation and slight disc height loss; there is disc protrusion (including a cystic component) with impingement to the left traversing S1 nerve root.
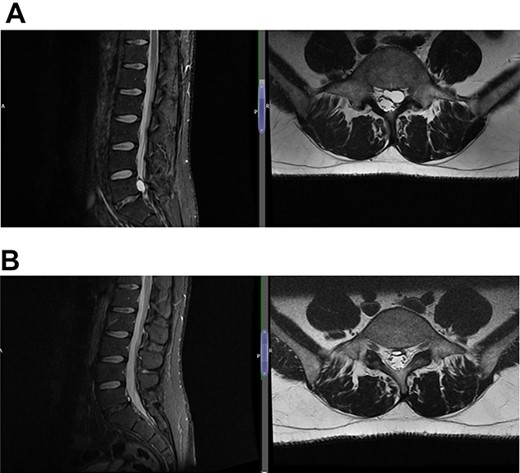
(A) Case 2—initial MRI shows a slightly unusual abnormality associated with the L5-S1 compressing the left transiting S1 nerve root and is almost certainly a fragment of extruded/sequestered disc, and (B) case 2—updated MRI shows a significant decrease in the previously seen cystic lesion at L5-S1 level now measuring 4 mm (AP diameter, previously 11 mm).
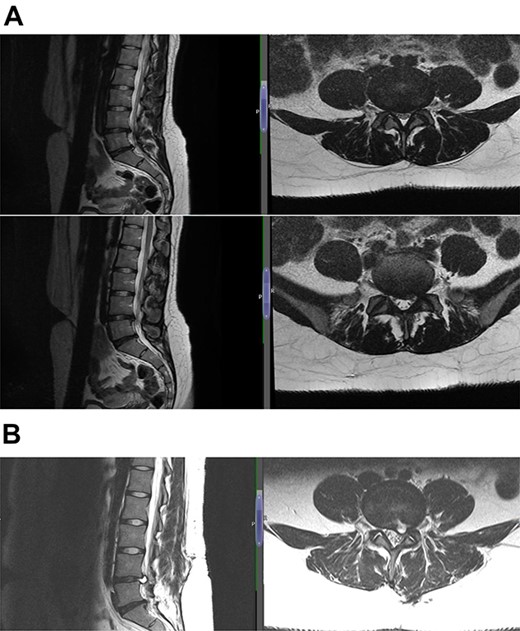
(A) Case 3—an early MRI demonstrates at L5/S1 level moderate diffuse disc bulge is seen with early bilateral facet joint degenerative changes; there is irritation of the L5 nerve roots bilaterally, and at L5/S1 level moderate left posterolateral disc bulge is seen irritating the S1 nerve roots bilaterally left more than right, and bilateral facet joint degenerative changes also noted at this level, and (B) case 3—a later MRI, the cystic changes noted at the L4-L5 level which clearly shows a connection with the intervertebral disc.

(A) Case 4—an initial MRI showing multilevel degenerative disc disease and a cyst at the lumbar L5/S1 which is causing impingement on the left S1 nerve root, and (B) case 4—comparison was made with the previous MRI; post-contrast scan shows that the L5-S1 left-sided cystic features have nearly diminished; no neoplastic lesion is detectable.
It would explain that young men are predominantly affected; this demographic is more likely to engage in risky behaviour and is more likely to be involved in or sustains high-impact injuries or trauma [2, 9, 12–14]. There is evidence to suggest that it is the resorption of disk fragment or that of the herniated material and the reactive process leads to that results in DC formation [9–11]. The insult regardless of the mechanism results in the formation of a reactive pseudo-membrane is supported by histology of the DCs showing no synovial lining cells, demonstrating fibrous connective tissue [9–11].
Another other prevailing hypothesis suggests that a disc herniation or disc injury (whether from the same forces as described above or not) leads to an acute haemorrhage of the epidural venous plexus causing an epidural haematoma and the cyst develops secondary to an impairment of haematoma resorption or mucous degeneration at the surface of the haematoma, encapsulating and later forming cysts [2, 9–14]. The earliest reported incidences of these cysts in the literature, from the 1990s, in which majority of the cysts investigated contained haemorrhagic fluid/haemosiderin [9, 10, 12, 15]. The haematoma formation secondary to the insult could be discal in nature, although it does not account for the formation of a direct communication between the cyst and the corresponding disc [10].
In this case series, it was noted on MRI, there is high intensity signal on T2 sequence in the facets along with fluid along the facet joint, a probable indicator of facetal micro-instability [16]. The MRI findings lend itself to the first hypothesis that repetitive stress and strain at the discal level, i.e. mechanical stress, accelerates the degenerative process in the disc leading to its cystic degeneration of an extruded disc segment. The act of microdiscectomy disturbs the posterior elements including the facets joints and, if not already present, can result in facet micro-instability. A recommendation to avoid any heavy lifting invariably reduces the mechanical stress and strain on the facet joint, the continued degeneration and evident in the case cohort of regression of symptoms.
CONCLUSION
Post-PDP and DC exhibit similarities in both histological and epidemiological characteristics, which indicates the same likely pathological origin of post-PDP and DC. For patients with mild to moderate symptoms, conservative treatment can lead to great improvement, even inducing spontaneous regression.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
REFERENCES
Author notes
N. Jadhav, L. Sivakumar and S. S. Talibi have contributed equally to this work.



