-
PDF
- Split View
-
Views
-
Cite
Cite
Stavros Chrysovalantis Liapis, Alexios Stavrou, Konstantinos Perivoliotis, Prokopis Christodoulou, Georgios Kalodimos, Georgios Kitsakis, Kassiani Kapatou, Dimitrios Ziamas, Dimitrios Lytras, Laparoscopic cholecystectomy for acalculous, gangrenous cholecystitis on an outpatient COVID-19 adult: a case report, Journal of Surgical Case Reports, Volume 2022, Issue 5, May 2022, rjac205, https://doi.org/10.1093/jscr/rjac205
Close - Share Icon Share
Abstract
We report the rare case of an acalculous, gangrenous cholecystitis on a, previously healthy, outpatient COVID-19 adult. The 53-year-old patient presented to the emergency department due to epigastric pain and fever and was admitted to the COVID-19 department. Due to clinical and laboratory deterioration, a computed tomography scan was performed that confirmed the diagnosis of acalculous cholecystitis. The patient was submitted to laparoscopic cholecystectomy. Intraoperatively, a gangrenous gallbladder was identified. Immunohistology validated the presence of specimen wall vasculitis and vessel thrombosis.
INTRODUCTION
SARS-COV-2 coronavirus resulted in the breakout of the recent COVID-19 worldwide pandemic. COVID-19 is associated with a considerable morbidity and mortality burden due to the development of acute respiratory distress syndrome and multiple organ failure. Most COVID-19 patients present with fever and respiratory symptoms; however, extrapulmonary symptoms are not uncommon.
Previous studies confirmed the biliary tropism of the novel coronavirus [1]. Literature reports suggested a more aggressive presentation of cholecystitis, regardless of the presence or not of cholelithiasis [1]. These associated biliary manifestations are considered to be the result of a dysregulated inflammatory response, rather than a direct viral injury [2, 3].
To the best of our knowledge, only a limited number of COVID-19 cases complicated with acalculous gangrenous cholecystitis have been reported [4–6]. Moreover, the development of this severe manifestation in outpatient COVID-19 patients with no previous comorbidities is an even rarer clinical entity [4–6].
Herein we present the case of an acute, acalculous, gangrenous cholecystitis in a previous healthy, outpatient COVID-19 patient.
CASE REPORT
A 53-year-old male, with no previous medical history and a BMI 33.9 kg/m2, was referred to the emergency department of our institution due to epigastric pain and fever. The patient had a positive COVID-19 polymerase chain reaction (PCR) test 10 days before current presentation, with multiple fever spikes (up to 38.2°C), mild dyspnea and nonproductive cough symptoms. During this period, he did not receive any medications, except paracetamol.
Vital signs upon presentation were the following: heart rate 95/min, blood pressure 150/90 mmHg, SpO2 95% and respiratory rate 18/min. There were no pathologic breathing sounds at auscultation, whereas physical examination of the abdomen revealed right upper quadrant tenderness and a positive Murphy’s sign.
Laboratory examinations highlighted a normal leukocyte count (WBC 10.70 K/μl) and C-reactive protein levels (CRP 5.49 mg/L). However, an abnormal biochemical liver profile (SGOT 52 IU/l, SGPT 119 IU/l, γ-GT 117 IU/I) and ferritin (486 ng/ml) was noted. Nasopharyngeal swab-PCR was positive for SARS-CoV-2 infection. Chest X-ray showed interstitial lung infiltrations, whereas abdominal ultrasound identified a moderate gallbladder wall thickness without the presence of pericholecystic fluid or gallstones (Fig. 1).
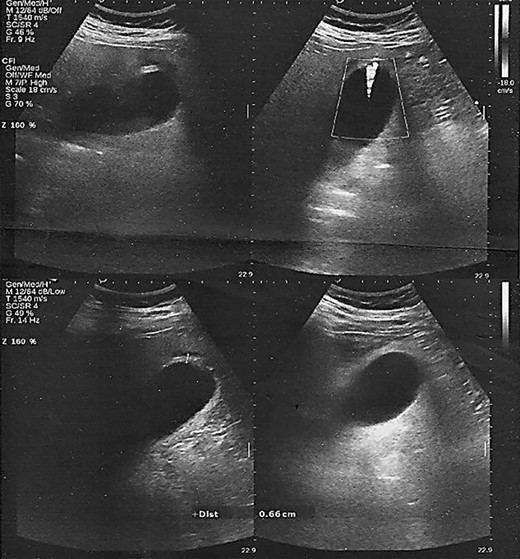
Ultrasound scan. Gallbladder wall thickening without the presence of pericholecystic fluid or gallstones.
The patient was, initially, admitted to the COVID-10 department and received empiric antibiotic coverage (Piperacillin and Tazobactam), thromboprophylaxis (enoxaparin 4000 IU) and bronchodilator agents (budesonide and ipratropium). Upon the third day of admission, due to the ongoing fever and the increase in the inflammatory markers (WBC 15.80 K/μl, CRP 297.07 mg/L, ferritin 821 ng/ml), a chest and abdominal computed tomography (CT) scan were performed. Imaging confirmed the presence of marked gallbladder dilatation and wall thickening, combined with free peritoneal fluid and pericholecystic omental involvement (Fig. 2).
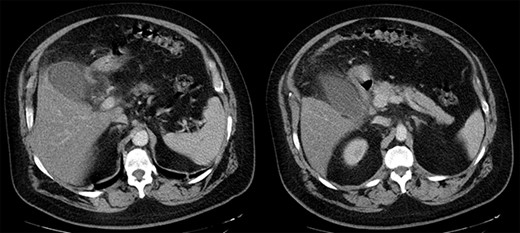
Abdominal CT scan. Marked gallbladder dilatation and wall thickening, combined with free peritoneal fluid and pericholecystic omental involvement.
After surgical consultation, the patient was submitted to a standard four-port laparoscopic cholecystectomy. Intraoperatively, a gangrenous gallbladder was identified, with no sign of perforation. Despite the disseminated inflammation, a critical view of safety was successfully achieved. Cholecystectomy was completed with the use of an energy device (Harmonic™). Operation duration was 140 min.
Postoperative recovery was uneventful, with a complete symptom remission. The patient was discharged on the third postoperative day. No complication was reported during the follow-up.
Bile COVID-19 PCR was negative. Histological findings of the specimen included extensive mural hemorrhagic infiltrates (Fig. 3). Immunochemistry showed inflammatory infiltrates and obliteration of the medium-sized arteriole lumen (CD31). Perivascular space involvement, an increased count of CD68 macrophages and CD4 lymphocytes was also identified. All these findings were indicative of acute gangrenous cholecystitis on the basis of gallbladder wall vasculitis and subsequent thrombosis.
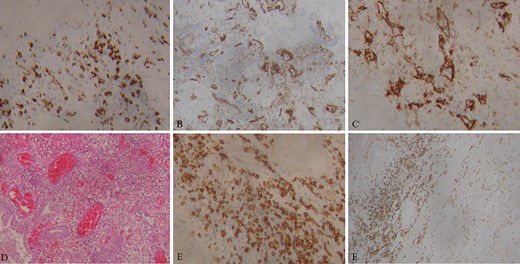
Images of immunohistology staining of gallbladder specimen. CD68 macrophages and fibroblasts (A). CD31 Medium-sized vessels with local inflammatory infiltrations (B, C). Hematoxylin–eosin-stained sections of the gallbladder; inflammatory infiltrates diffusely involve medium-sized vessels, with lumen obliteration (D). CD4 Lymphocytes (E, F).
DISCUSSION
Acalculous gangrenous cholecystitis is a severe clinical condition, commonly found in critically ill or immunosuppressed patients [6]. The main pathophysiological pathway includes bile stasis due to gallbladder hypomotility, increased intraluminal pressures, and subsequently, ischemia and necrosis [7]. Despite the low incidence, it is frequent after major trauma, sepsis, prolonged parenteral nutrition and extended surgical resections [7].
Acalculous gangrenous cholecystitis has been, also, reported in COVID-19 patients. Although most cases consist of septic or under mechanical ventilation patients, there are reports of mild-to-moderate COVID-19 patients that developed acute cholecystitis; in fact, current literature suggests a more aggressive disease in infected patients [1].
The exact mechanism, though, is still unclear. SARS-COV-2, through the protein Spike (S), binds to the angiotensin-converting enzyme 2 receptors which are expressed in many organs, including biliary and gallbladder epithelial cells [8, 9]. However, there is still a debate whether a direct viral injury is responsible for the clinical condition; this is also supported by the fact that, like in our case, in many reports, bile tests were negative for viral load [10].
Another hypothesis is based on coagulation dysregulation that results in gangrenous cholecystitis development in COVID-19 patients [11]. More specifically, SARS-COV-2 augments endothelial cells’ overexpression, thus resulting in lumen obstruction and mural necrosis. Moreover, the consequent release of proinflammatory cytokines, including interleukin-6 and tumour necrosis factor alpha, promotes the coagulation cascade [11]. Indeed, the onset of disseminated intravascular coagulation (DIC) in COVID-19 is well documented; almost 70% of mortally ill patients display DIC signs [11].
Immunological derangement is a crucial part of COVID-related cholecystitis. Case series validated that symptom onset aggregates at 17–24 days following SARS-COV-2 infection [2]. This timing is in accordance with the development of the Multisystem Inflammatory Syndrome in Children and adults [2].
Current guidelines retain laparoscopic cholecystectomy as the gold standard treatment for COVID-19 cholecystitis patients. Initial concerns regarding viral dissemination through aerosolization have not been confirmed [12, 13]. In previous cases, a percutaneous cholecystostomy approach has been described [7]; however, drainage is retained for critically ill patients and is contraindicated in cases of gallbladder ischemia [12, 13].
This report describes the case of a young outpatient COVID-19 male, with no previous comorbidities that acutely developed acalculous gangrenous cholecystitis and was successfully submitted to laparoscopic cholecystectomy. Due to the insidious and rapid progression of this clinical entity, an increased degree of clinical suspicion and close monitoring is required to provide the appropriate therapeutic modality.



