-
PDF
- Split View
-
Views
-
Cite
Cite
Ummey Hani, Quratulain Tariq, Amman Bari, Saad Bin Anis, Asad Diwan, Benign pituitary adenoma with multiple intracranial metastases—a case report and review of the literature, Journal of Surgical Case Reports, Volume 2022, Issue 5, May 2022, rjab382, https://doi.org/10.1093/jscr/rjab382
Close - Share Icon Share
Abstract
Metastatic benign pituitary adenomas (PAs), also known as pituitary carcinomas (PCs), represent 0.1–0.2% of all intracranial lesions. They are rare and challenging pathologies. We present the case of a 34-year-old female, who presented to the clinic with headache and visual loss. She was diagnosed with PA with multiple extra-axial metastases. Debulking and biopsy of the lesions was done. Postoperatively, there was gross residual disease, and the patient’s visual disturbances did not improve. Only 165 cases of PCs have been reported in the current literature. Existing consensus on management of these uncommon lesions is based on trial and previously published case reports and surgery appears to be the only definitive treatment. Further research regarding any non-surgical expectant management is warranted.
INTRODUCTION
Pituitary adenomas (PAs) are the third most common intracranial lesions [1]. Their malignant counterpart, defined by metastasis from a primary, histopathologically benign lesion, is, however, uncommon [1, 2]. Also known as pituitary carcinoma (PC), the latter comprise only 0.1–0.2% of intracranial lesions [2, 3]. Theoretically, surgery for the primary lesion plays a major part in the pathophysiology since it is not infrequent to enter the subarachnoid space in process [4]. Owing to its rarity, neurosurgeons have largely struggled with its diagnosis and management [1]. In this report, we present the case of a patient with non-functioning PA with multiple intracranial metastases, who underwent two surgeries in a year period.
CASE PRESENTATION
A 34-year-old female presented with 20 days of symptoms including headache, dizziness and visual deterioration. Her past medical and surgical history was notable for resection of PA via a right frontal craniotomy, 10 years’ prior, followed by radiation of the residual disease. Neurological examination was notable for complete loss of vision in the right eye and temporal hemianopia in the left. Other cranial nerves were intact, and the rest of the examination was unremarkable.
Brain magnetic resonance imaging (MRI) demonstrated a 3.1 × 4.6 × 5.2 cm (transverse × antero-posterior (AP) × craniocaudal (CC)) heterogeneously enhancing lesion in the sellar region, with suprasellar extension and involvement of the clivus (Fig. 1A and B). Posterior displacement of the left cerebral peduncle, corticospinal tracts and the left middle cerebellar peduncle by the lesion was also noticed. However, there was no evidence of the lesion infiltrating these tracts. Also demonstrated on the MRI was another abnormally enhancing, extra-axial lesion, 1.6 × 1.6 × 1.3 cm (transverse × AP × CC), just above the foramen magnum, on the right side of the midline, anterior to foramen of Luschka. There was no evidence of extension of this lesion to the brain parenchyma.
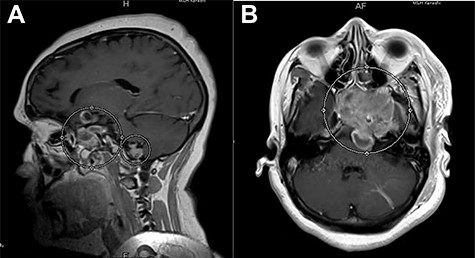
(A) Post-contrast T1-weighted MRI, sagittal section, showing a heterogeneously enhancing lesion in the right sellar region, with supra-sellar extension, and clivus involvement. (B) Post-contrast T1-weighted MRI, axial section, showing re-demonstration of abnormally enhancing lesion with superior cystic component just above the foramen magnum on the right side of the midline.
The patient was scheduled for neuro navigation-guided left frontal craniotomy and debulking of the sellar lesion. She was positioned supine, with head fixed in a three-pin Mayfield clamp. A left frontal incision was given, and craniotomy, performed. Multiple grey–white dural-based nodules, found on removal of the bone flap, were biopsied. A D-shaped durotomy followed, and the frontal lobe was retracted. The lesion was identified as an encapsulated, suck-able tumor, with a hard capsule, moderately vascular and encroaching both the internal carotid arteries and optic nerves bilaterally. The tumor was debulked by removing it piece-meal. The specimen was sent for histopathological examination in two containers, one containing a piece of the sellar tumor, and the other containing multiple tissues taken from the frontal dural-based nodule. Histopathological examination (HPE) of both revealed a neoplastic lesion arranged in the form of nests and papillary architecture, with tumor cells arranged around fibrovascular cores (Fig. 2A and B). There was no evidence of malignancy, thereby confirming the diagnosis of PA.
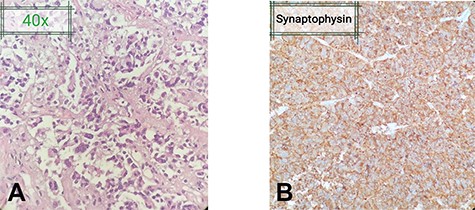
(A) Fragments of the lesion arranged in the form of nests and papillary architecture with tumor cells arranged around fibrovascular cores. The tumor cells are monomorphic, have moderate amount of cytoplasm and nuclei showing stippling of chromatin. (B) Tumor cells highlighted on immunehistochemical stain Synaptophysin. No significant nuclear atypia or mitoses are appreciated.
Postoperatively, the patient’s vision did not improve, and her left eye was medially deviated, on examination. She was managed in special care, with endocrinology on-board. Immediate postoperative MRI showed heterogeneously enhancing residual lesion in the right sellar region with suprasellar extension, with areas of postsurgical subdural hemorrhage. Abnormally enhancing lesions in the posterior fossa were also re-demonstrated. There were no complications, and the patient was discharged as scheduled. At her last follow-up in August 2020, the patient had stable disease without any further deterioration.
DISCUSSION
PCs are poorly understood pathologic entities, rarely described previously in the literature. Metastasis is the defining criterion that differentiates an atypical adenoma from a PC [2, 5, 6]. In the literature, while no sex proclivity is observed [5, 6], a predilection for adults in their fifth decade of life is reported [5]—unlike our case, where the patient was in her thirties. Although dural invasion has been shown to be more common in non-functioning adenomas [7], functional lesions form the bulk of dural metastasis [8, 9]. Endocrinologically inactive lesions are reportedly the rarest type of PCs [1, 3, 5, 6, 10]—our patient being a case-in-point because her tumor exhibited normal endocrine parameters. Common sites for metastases include cerebral cortex, posterior fossa, meninges, liver and lungs, with a greater reported frequency of systemic metastases than craniospinal metastases [2, 3, 5, 6]. Natural progression of an adenoma into a carcinoma may vary from months to years, with 43 years being the longest latency interval communicated in the literature [5, 6]. An overall mean reported interval to metastasis was 4.7–6.6 years [3, 5, 6], which usually included either drop metastasis to the spine or distant metastases to liver and bone [8, 9, 11, 12]. This was not comparable to how our patient presented, with intracranial metastases occurring a decade after craniotomy for the resection of their benign counterpart.
Unlike our case, most metastases display nuclear atypia and mitoses. Potential biomarkers are being increasingly, but superficially, explored, with most PCs reportedly exhibiting higher Ki-67 and p53 indexes [2, 5, 10]. Mutations in H-Ras and Rb gene, as well as acquired cell-immortality with increased telomerase and hTERT expression, have been observed [5, 10]. Also reported is a correlation of tumor cell cycle progression, angiogenesis, metastasis and invasion with upregulation of cyclin D1, VEGF and miRNAs, as well as of apoptosis, tumor suppression, metabolism and enhanced temozolomide sensitivity with downregulation of MGMT and p16Ink4A [3, 13]. However, any comparisons with our patient could not be made in this regard due to the unavailability of molecular testing in Pakistan. Of several postulated theories for metastases, protracted treatment, especially craniotomy, was the commonest [1, 2, 5–7]. This is due to iatrogenic injury to the arachnoid or vessels, resulting in subarachnoid or hematogenous seeding, respectively [6]. Other reasons included dissemination by surgical instruments or flush water, postoperative irradiation, cancer dormancy leading to mutations and de-novo tumorigenesis. However, no definitive correlations were made [1, 5, 6].
Presenting symptoms, comparable to our patient’s, include visual disturbances, headache and/or hyper/hypopituitarism [2, 5]. Brain MRI is the best diagnostic tool [10], but the efficacy of PET and radionucleotide scintigraphy is being tested for functional carcinomas [5]. Complete resection via the transcranial route remains the treatment of consensus, followed by adjuvant radiation and chemotherapy for residual disease. Temozolomide, along with 5-ALA fluorouracil are commonly used chemotherapeutic agents [1–3, 5]. However, treatment is largely palliative, and the prognosis is poor [5, 10]—66% of patients die within 1 year [5]. This again makes our patient a case-in-point, who was still living with stable disease, 2 years after her diagnosis and treatment.
CONCLUSION
Significant research in targeted molecular and immunotherapy, and well as pre- and post-surgery surveillance, will allow for effective management and better patient outcomes in these deadly neoplasms.
CONFLICT OF INTEREST STATEMENT
None declared.
AUTHORS’ CONTRIBUTIONS
UH: Literature review, developing and finalizing the manuscript. QT: Study conceptualization, manuscript development. AB: Manuscript development. SBA: Study supervision and conceptualization, approval of the final manuscript.
DISCLOSURES
The authors have no disclosures to report. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This manuscript is a unique submission and is not being considered for publication, in part or in full, with any other source in any medium. All authors agree with these terms.



