-
PDF
- Split View
-
Views
-
Cite
Cite
Minglei Zhong, Zhaocun Zhang, Wenqiang Qi, Yongheng Zhou, Guangda Lv, Xianzhou Jiang, Mucin-poor mucinous tubular and spindle cell carcinoma of the kidney: one case report and review of the literature, Journal of Surgical Case Reports, Volume 2022, Issue 4, April 2022, rjac185, https://doi.org/10.1093/jscr/rjac185
Close - Share Icon Share
Abstract
Mucinous tubule and spindle cell carcinoma (MTSCC) of the kidney is a rare renal pleomorphic tumor considered as low-grade malignant, with occurring mainly in female. Few mucin-poor MTSCC cases have been reported so far. A typical MTSCC is composed of closely arranged tubules with pale mucus matrix and spindle cell components. Mucin-poor MTSCC is difficult to distinguish from other renal cell carcinomas due to small amount of mucus. We reported a case of mucin-poor MTSCC in a 37-year-old male with detailed imaging, histology, immunohistochemical and next-generation sequencing information, looking forward to providing an insight into mucin-poor MTSCC.
INTRODUCTION
Mucinous tubule and spindle cell carcinoma (MTSCC) of the kidney firstly reported by Ordofiez in 1996 [1] is a unique subtype of renal cell carcinoma. MTSCC was firstly described as an independent and relatively rare subtype of renal tumors in the 2004 World Health Organization classification of adult renal epithelial neoplasms [2]. It affects more female than male [3]. The age at onset is spread over a broad range, and it is a tumor primarily of adulthood. Nephron-sparing surgery is the first choice in many literatures based on its relatively good prognosis. This paper reported a case of mucin-poor MTSCC and reviewed relevant literatures. The diagnosis of the case mainly relies on histopathology and immunohistochemistry.
CASE REPORT
A 37-year-old male underwent a computed tomography scan (CT), which revealed a 2.5 × 1.9 cm slightly lower density ingrown mass in the lower pole of left kidney for 1 month. The patient was asymptomatic and had a history of kidney stones. The shape of the mass was regular, with unclear boundaries. Enhanced CT showed that the enhancement of the mass was lower than that of the surrounding renal parenchyma (Fig. 1). On magnetic resonance imaging (MRI), a circular abnormal signal was detected in the cortical medullary area of left kidney, with an equal signal on T1WI and a slightly low signal on T2WI (Fig. 2A and B). The enhanced MRI scan showed no obvious enhancement in the cortical and medullary phases and a slightly dotted high signal in the excretion phase (Fig. 2C–F). The mass was a space-occupying ingrown lesion with insufficient blood supply. Patients underwent laparoscopic partial nephrectomy and received no postoperative therapy. After the tumor was incised, a 2.3 × 2 cm soft mass that was grayish-white and slightly grayish-yellow was found with clear boundary. Histology (hematoxylin and eosin staining) results showed that the tumor was composed of elongated tubules arranged in parallel. The tubules lined with cuboid and spindle cells set in a small amount of myxoid matrix (Fig. 3A). Most of the tumor cells were round, a few were spindle and elliptical, with eosinophilic nucleoli. Mitotic figures were rare and the atypia is not obvious. Obvious foamy macrophage aggregates could be seen in some areas (Fig. 3B). Immunohistochemical results were as follows: CK7(+), P504S (+), PAX-8(+), CD10(−) (Fig. 3C–F). The Ki67 stain showed proliferation rates up to 5%. The pathological result demonstrated left renal mucin-poor MTSCC. We performed next-generation sequencing (NGS) for case, and the result showed that the tumor mutational burden was 2.74 mutation/Mb. RET gene mutation was considered as a mutation of uncertain significance. The detection of multiple genetic polymorphism sites of tumors shows that it may be slightly sensitive to some chemotherapeutics like gemcitabine. PD-1/PD-L1 immunotherapy may not benefit the patient. The patient was followed up for 8 months after surgery. No evidence of metastasis or recurrence was found.
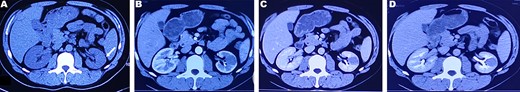
CT plain scan and enhanced scan of mucus-poor MTSCC. (A) CT scan showed a low-density mass in the lower pole of the left kidney (arrow), unclear boundary, swelling growth in kidney; (B) enhanced CT scan of corticomedullary phase shows tumor mild homogeneous enhancement, (C) nephrography phase, (D) renal excretion phase.
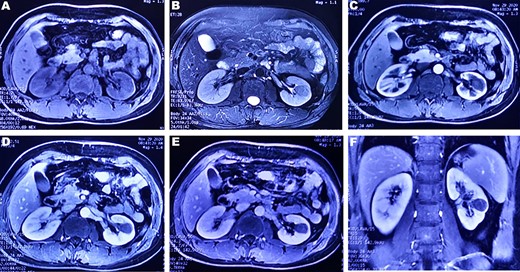
(A and B) A round-like abnormal signal can be seen in the cortex and medulla of the left kidney, which is equal signal on T1WI and slightly low signal on T2WI; (C–E) In the arterial phase, parenchymal phase and venous phase of enhanced MRI, a heterogeneous, progressive and slow enhancement is noted. (F) Enhanced arterial phase of coronary T1WI showed a low signal mass located in the lower pole of the left kidney.
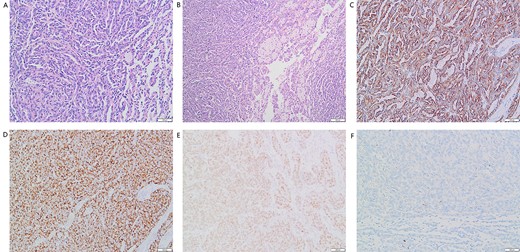
(A) Many elongated tubular epithelial cells arranged in a small amount of mucus. Some spindle cells can be seen (magnification, ×20); (B) Obvious foamy macrophage aggregates in some areas (magnification, ×10). Immunohistochemical results: (C) CK7(+), (D)P504S (+), (E)Pax-8(+), (F)CD10(−).
DISCUSSION
MTSCC was classified into ‘classic’ and ‘mucin-poor’ based on the proportion of interstitial mucin in histology [4]. Few studies individually described the imaging characteristics of mucin-poor MTSCC. In all phases of enhanced CT, the mean attenuation of MTSCC tumor was not only less than the normal renal cortex and medulla, but also less than collecting duct carcinoma [5–7]. The tumors presented homogeneous pattern enhancement and were usually small (≤5 cm) [6]. In MRI scans, the T1WI signal was isointense to normal renal parenchyma. T2WI signal was usually diverse, including hypo-, iso- and hyperintensity [5]. In our study, tumors presented low-signal intensity on T2w images. This may be related to no mucinous or myxoid stroma. It may be a significant feature to distinguish mucin-poor MTSCC and classic MTSCC on imaging diagnosis.
According to previous reports, the MTSCC origin is controversial. It may originate from the loop of Henle, collecting duct or the proximal tubule [8]. In our study, AMACR(P504S) were positive. This seems to support the origin of the proximal tubule. However, immunohistochemical features are difficult to reveal the origin of MTSCC [9].
MTSCC is primarily diagnosed by postoperative histology and immunohistochemical evaluation. But the diagnosis is becoming more difficult due to the expansion of the histologic spectrum of it. There are some overlapping features between MTSCC and type 1 papillary renal cell carcinoma (PRCC), including focal compressed tubule papillary areas, aggregates of foamy macrophages, mucinous material and some features of immunohistochemistry [8]. However, compared with PRCC, MTSCC usually lacks the gains of chromosomes 7 and 17 and losses of chromosome Y [10]. In order to make a more accurate diagnosis, interphase fluorescence in situ hybridization is available as an option.
Partial or radical nephrectomy is the standard treatment of MTSCC, with a favorable prognosis usually. It is recommended to spare the involved kidney when MTSCC is suspected. Sarcomatoid change or high-grade histological features seem to be related to recurrence or metastasis [11]. A few mucin-poor MTSCCs reported in recent years were accompanied by recurrence and metastasis [12]. The case in present study has not found evidence of disease progression, but the postoperative patient still needs to be closely observed and followed up.
For progressive tumors, there is no standard adjuvant treatment currently. Patients with widespread metastatic MTSCC may be responsive to sunitinib [6, 13]. Hippo pathway dysregulation plays a key role in the pathogenesis of MTSCC [14]. This may provide a basis for its further diagnosis and treatment. In present study, we performed NGS for the patient. No pathogenic or potentially pathogenic genetic variant has been detected in this patient. The result of NGS indicated that chemotherapy and immunotherapy have little therapeutic value for our patient. This may provide a reference for the later treatment of the patient.
In conclusion, the imaging characteristics of mucin-poor MTSCC are not significantly different from those of classic MTSCC. A slightly low signal on T2WI may be a feature to distinguish them. According to immunohistochemistry and genetics, it can be better differentiated from other types of kidney tumors. General treatment is radical nephrectomy or partial nephrectomy and most patients have a good prognosis. Genetic testing may provide more personalized treatment.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
Natural Science Foundation of Shandong Province (ZR2020MH082).



