-
PDF
- Split View
-
Views
-
Cite
Cite
Leonid A Belyayev, Sophia M Foroushani, Daniel C Wiener, Westyn Branch-Elliman, M Blair Marshall, Hassan A Khalil, Bronchopleural fistula due to cavitary pneumonia after SARS-CoV-2 infection treated with open thoracostomy, Journal of Surgical Case Reports, Volume 2022, Issue 4, April 2022, rjac076, https://doi.org/10.1093/jscr/rjac076
Close - Share Icon Share
Abstract
Severe coronavirus disease of 2019 (COVID-19) disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causes substantial parenchymal damage in some patients. There is a paucity of literature describing the surgical management COVID-19 associated bronchopleural fistula after failure of medical therapy. We present the case of a 59-year-old woman with SARS-CoV-2 pneumonia, secondary bacterial pneumonia with bronchopleural fistula and radiographic and clinical evidence of disease refractory to medical therapy. After a course of culture-driven antimicrobial therapy and failure to improve following drainage with tube thoracostomy, she was treated successfully with Clagett open thoracostomy. After resolution of the bronchopleural fistula, the thoracostomy was closed and she was discharged home. In cases of severe COVID-19 complicated by bronchopleural fistula with parenchymal destruction, a tailored approach involving surgical management when indicated can lead to acceptable outcomes without significant morbidity.
INTRODUCTION
Severe coronavirus disease of 2019 (COVID-19) disease is associated with direct lung injury and secondary lung infections. Bacterial pneumonia complicates ~25% of mechanically ventilated patients with COVID-19 [1–4]. Staphylococcus aureus is a common cause of ventilator-associated pneumonia that can lead to a severe fibrinopurulent reaction and empyema, parenchymal destruction and air leak. Despite several reports of lung necrosis necessitating salvage operations, there are no reports on open thoracostomy drainage for the treatment of COVID-19 associated bronchopleural fistula after failed conservative management [5].
CASE REPORT
We present a case of a 59-year-old woman with no smoking history and past history notable for hyperlipidemia and migraines who presented to a community hospital with symptoms of fatigue, malaise and shortness of breath. She was not vaccinated for COVID-19. She tested positive for COVID-19 by polymerase chain reaction. She was treated with casirivimab and imdevimab monoclonal antibodies in the outpatient setting but continued to have fever, persistent cough and shortness of breath. Given these symptoms, she was treated with a course of amoxicillin-clavulanic acid and prednisone in the ambulatory setting for presumed bronchitis.
Three weeks following her initial COVID-19 diagnosis, she presented to the hospital with ongoing symptoms and was found to have a right hydropneumothorax and cavitary lesion with parenchymal destruction (Fig. 1). She was treated with broad-spectrum antibiotics and percutaneous chest tube drainage, which showed a continuous one- to two-column air leak. Her pleural fluid grew methicillin-sensitive S. aureus (MSSA). Bronchoscopy revealed mucosal edema and erythema, particularly in the right upper lobe. Despite treatment, she had a persistent air leak, and she was transferred to our facility for further management.
At the time of transfer, the patient was afebrile and hemodynamically normal, and she was not requiring supplemental oxygen. Her C-reactive protein level was 14.86 mg/l (reference <10) and white blood cell count was 8800 per mm3 (reference 4.5–10). Prior to transfer, her C-reactive protein level had been within normal limits. Computed tomography (CT) of the chest showed a residual pneumothorax (Fig. 2) and peripheral bronchopleural fistula (BPF). We treated her with open thoracostomy drainage (Clagett procedure), removing a segment of the second rib and suturing skin flaps to the thickened parietal pleura (Fig. 3). Due to institutional policy, no intra-operative images were obtained. On direct visualization, the visceral pleura overlying the upper lobe was thickened, but no frank purulence was noted. There was fibrinous exudate and air leak at two sites on the surface of the lung. A sample of the fibrinous tissue was sent to microbiology for clinical culture, which subsequently grew MSSA.
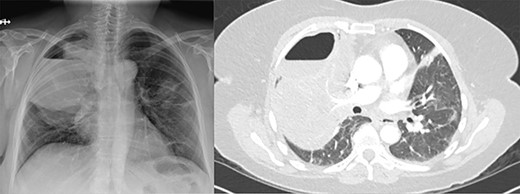
(A) Plain film of chest showing apical hydropneumothorax. (B) Computed tomography axial image demonstrating a complex hydropneumothorax with significant right sided parenchymal infiltrate.
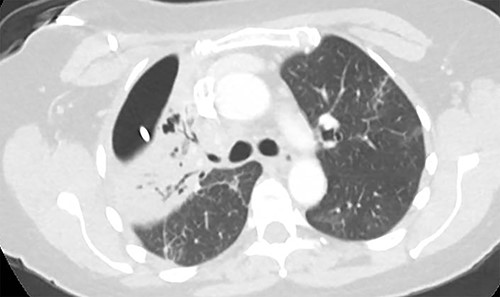
Axial image demonstrating right upper lobe consolidation and residual pneumothorax despite tube thoracostomy.
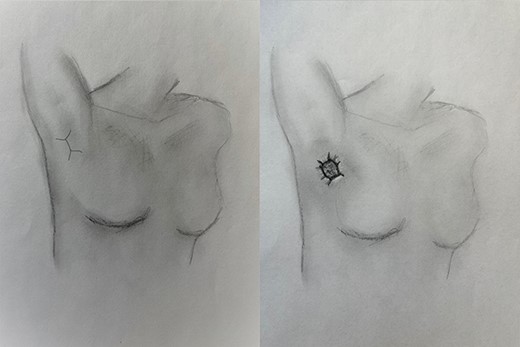
(A) Location of incision for Clagett open thoracostomy, and (B) maturation of thoracostomy with skin flaps.
The wound was managed with −75 mmHg negative pressure wound therapy and bedside dressing changes twice per week. Within 4 days, the air leak resolved, and we considered a latissimus dorsi muscle flap for closure of the defect. We repeated a CT for operative planning. This showed a small space and no residual fluid collections. Ten days following the Clagett procedure, we explored the cavity in the operating room and found healthy granulation tissue with a limited residual space and decided that a muscle flap was not needed. The Clagett window was closed in layers over a 19Fr channel drain. The drain had minimal serous output and was removed 3 days later.
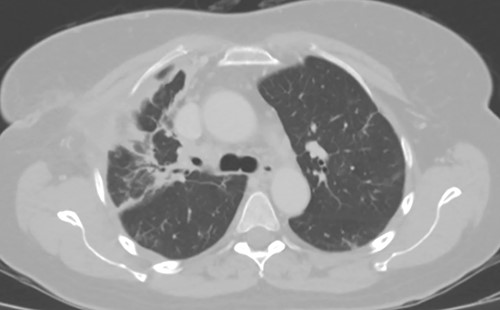
As the patient lived several hours away from the closest hospital, we obtained a second post-operative chest CT to ensure that she had no additional collections and to establish a new baseline. This demonstrated a 4 cm collection in the subcutaneous space. We aspirated the fluid, which showed no growth. The patient was discharged on a course of oral moxifloxacin. A repeat chest CT 4 weeks after Clagett closure was obtained and showed improved aeration of the right upper lobe (Fig. 4).
DISCUSSION
Secondary pulmonary bacterial infection is a known cause of significant morbidity for patients recovering from severe acute respiratory syndrome coronavirus 2 pneumonia [3]. Staphylococcal infections are a well-recognized source of empyema and lung necrosis with abscess formation. However, the natural history of these infections in the setting of COVID-19 is not well known. Reports of complications of necrotizing or cavitary infections, such as bronchopleural fistula formation, are also sparse in the literature. Although nonoperative options including tube thoracostomy can be successful, open thoracostomy drainage remains a critical element in the thoracic surgeons repertoire for the management of significant lung parenchymal destruction and empyema when the lung fails to expand, especially with persistent air leak from a bronchopleural fistula [5, 6]. Key tenets of open thoracostomy include control of sepsis through wide drainage to create an aperture facilitating frequent dressing changes either in the form of wet-to-dry gauze or negative pressure wound therapy. This approach also allows for removal of fibrinous deposits on the lung promote formation of granulation tissue and spontaneous closure of bronchopleural fistulae.
Closure of the Clagett space can be facilitated in a variety of ways to include primary closure over drains, pedicled flap closure (omental, myofascial) or thoracoplasty with collapse of the chest wall [7]. The fundamental principle is obliteration of empty space after surgical sterilization of the cavity. Use of endobronchial valves has been described in the treatment of bronchopleural fistulae, though in our opinion would need to be done judiciously and in combination with a strategy to manage the infected pleural space [8, 9]. Despite the majority of super-infections occurring in critically ill patients with COVID-19, a minority may present similarly to our patient with progressively worsening focal parenchymal disease. A tailored approach with aggressive management of sepsis, nutritional support and characterization of the underlying pathology can lead to acceptable outcomes with minimal morbidity.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



