-
PDF
- Split View
-
Views
-
Cite
Cite
Omar Marghich, Benjamin Benichou, Efoé-Ga Yawod Olivier Amouzou, Alexandre Maubert, Jean Hubert Etienne, Emmanuel Benizri, Mohamed Amine Rahili, Case series of mesh migration after rectopexy surgery for rectal prolapse, Journal of Surgical Case Reports, Volume 2022, Issue 2, February 2022, rjac017, https://doi.org/10.1093/jscr/rjac017
Close - Share Icon Share
Abstract
Mesh rectopexy for rectal prolapse can cause some serious mesh-related complications. Mesh migration into close viscera following rectopexy is rare. We report three cases of mesh migration after mesh rectopexy treated in our unit. The first patient presented with purulent discharge from the buttock 15 years after the rectopexy, the second patient presented with abdominal pain and pneumaturia also 15 years after the rectopexy and the third patient presented 22 years after the rectopexy with vaginal discharge. Diagnosis was made by physical examination, computed tomography scan, magnetic resonance imaging, cystoscopy or rectoscopy. The three patients underwent total removal of the meshes without any complications.
INTRODUCTION
While mesh rectopexy is considered to be an effective method for treating rectal prolapse, it can cause some serious mesh-related complication, such as mesh migration into close viscera. Herein, we report three cases of mesh migration following mesh rectopexy into the buttock, into both the rectum and the urinary bladder and into the vagina.
CASES PRESENTATION
Case N1
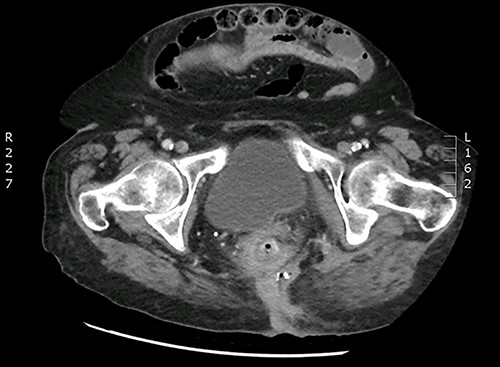
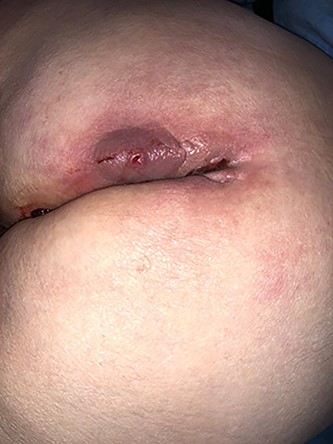
An 84-year-old woman underwent a posterior laparoscopic mesh rectopexy 15 years ago. In her past medical history, 4 years after the rectopexy, she had pelvic peritonitis of unknown origin (possibly linked to a mesh infection) for which she had a left iliac colonic stoma. The patient presented with perianal pus discharge. Our clinical exam showed an abscess with pus discharge in the left buttock. Rectum and vaginal examination did not reveal any abnormality. A computed tomography (CT) scan and a magnetic resonance imaging were performed showing changes suggestive of a mesh infection associated with an important inflammation and infiltration and a suspicion of a fistula in the rectum (Fig. 1).
The surgical procedure consisted of ablation of the rectopexy mesh by enlargement of the laterosacral fistula, with identifying the presence of a 4 mm fistula in the posterior wall of the rectum that was resected (Figs 2–4). The postoperative outcomes were uneventful.
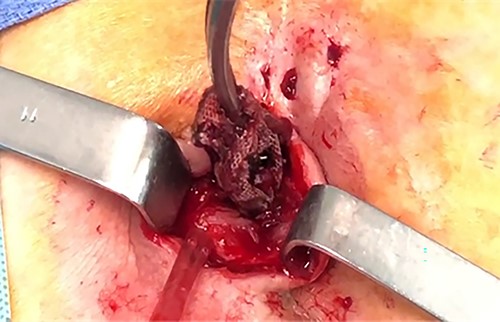
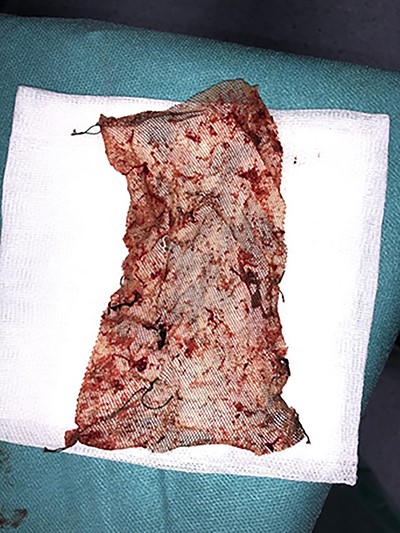
Case N2
A 90-year-old woman presented with abdominal pain and pneumaturia; in her medical history we find a laparotomy mesh rectopexy 15 years ago. CT scan showed inflammation around the mesh that can also be seen entering the bladder (Figs 5 and 6).
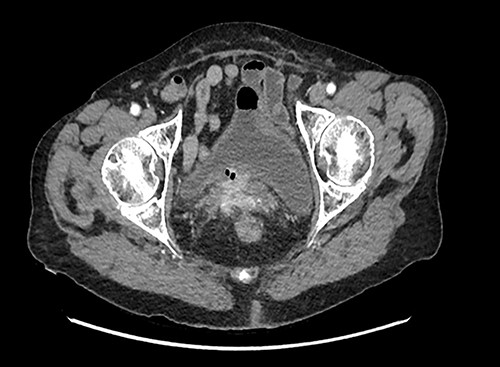
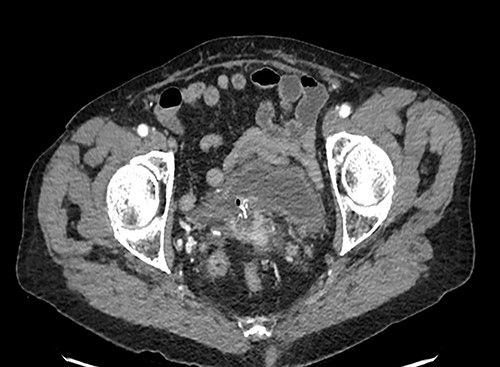
While cystoscopy showed the presence of the mesh in the urinary bladder, rectoscopy visualized the mesh on the anterior wall of the rectum at 3 cm from the anal marge.
The patient underwent a total removal of the mesh by transanal approach associated with a lateral sigmoidostomy with stapling of the efferent loop (Fig. 7).
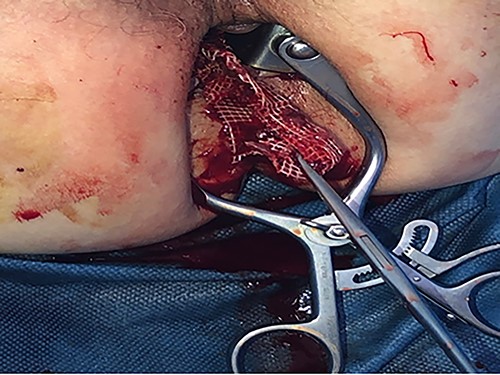
Control cystoscopy confirmed the total removal of the mesh. The patient had an indwelling urinary catheterization. A cystogram carried out after 6 weeks showed the persistence of the fistula in the bladder, so the patient kept the urinary catheter. Another cystogram 6 weeks later was normal; therefore, the catheter was removed. One year later, an opacified CT scan and a rectoscopy were undertaken and showed no fistula or other anomaly on the rectum; subsequently, the patient underwent a restoration of bowel continuity.
Case N3
A 42-year-old woman was admitted to our unit with vaginal discharge for 1 month. She had previously undergone an open anterior mesh rectopexy for complete rectal prolapse in 1997; she also had a history of mesenteric ischemia with an extensive intestinal resection in 2005. Vaginal examination showed the polypropylene mesh protruding out through the bottom of the vagina. Digital rectal examination was normal. CT scan showed an infection with an abscess of the rectopexy mesh, inflammation and infiltration next to the lower rectum and the anorectal junction fusing into the right ischioanal fossa. It also showed a vaginal fistula at the level of the posterior vaginal fornix, with an exposure of the mesh at this level. Under general anesthesia in gynecologic position, a sub-total removal of the mesh was done using a transvaginal approach. The postoperative outcomes were uneventful.
DISCUSSION
Rectal prolapse is a condition most commonly seen in elderly woman, and it is related to particular risk factors such as laxity of the pelvic floor muscles, abnormal fixation of the rectum or depth of the Douglas pouch [1].
Mesh rectopexy is a frequently performed surgery for the treatment of rectal prolapse. Presently, the laparoscopic approach is preferred as it has less postoperative pain, shorter hospital stay, and similar recurrence and morbidity rate when compared to open rectopexy [2]. In the other hand, robotic ventral rectopexy has emerged as a safe option compared to the laparoscopic approach with a trend towards a reduction in length of inpatient stay and postoperative complications [3].
With the more frequent use of mesh in pelvic organ prolapse surgery, new complications have been reported. Immediate, short-term and long-term complications have been reported in 2–16% [2], but the most serious complications of rectopexy are those related to the mesh itself like infection, extrusion, erosion and migration, reported in 4.6% [4].
While mesh migration into the rectum and the vagina have been reported in several articles, mesh migration into the buttocks is extremely rare.
In the literature, we found that the reported delay between mesh rectopexy and clinical symptoms of mesh erosion ranges from 4 to 124 months [5] and the clinical presentation of patients with mesh erosion can be very diverse and depends on the organ involved [2]. In our case series, all the symptoms of mesh migration were pronounced after more than 180 months.
Risk factors for erosion and migration are both mesh and patient related. In a systematic review, Balla et al. [1] reported that those complications are more frequent using synthetic material compared with biological mesh (mesh-related erosion rates were 1.87 and 0.22%). We also find that mesh-related complications are reduced using absorbable sutures compared with nonabsorbable sutures when performing laparoscopic ventral mesh rectopexy with synthetic mesh [6]. Certain surgical technical errors like unrecognized rectal injury and deeper stitches through the rectum may also contribute to mesh erosion [2]. Patient-related factors such as tobacco use, steroid intake, poorly controlled diabetes mellitus, Crohn’s disease, diverticular disease and prior pelvic radiation can cause mesh erosion and migration [1, 2, 4–7].
In our series, mesh migration was always related to the use of a polyester mesh and none of our patients had any other risk factors.
CT scan of abdomen and pelvis with contrast helps in localizing the mesh as well as the presence of any pelvic collection, severity of inflammation around the area of mesh and erosion into adjacent viscera [2].
Since the erosion of mesh into rectum is very rare, there is no well-defined protocol available in the literature for its management and it should be individualized for each patient based on location of erosion, severity of mesh protrusion into rectal lumen, presence of infection and degree of fibrosis around the area of mesh [1, 2, 4–8].
In all our three patients, a total mesh removal was performed without organ resection through the lumen of the concerned organ, no complication procedure-related and with a good postoperative follow-up.
CONCLUSION
Mesh migrations can occur very late after rectopexy, which makes it very difficult to link it to the rectopexy. Furthermore, elderly people with cognitive disorders do not know how to express symptoms, which are often not very specific. Thus, the mesh migration should be considered for patients with history of rectopexy presenting abdominal or vaginal symptoms. The use of biological mesh and absorbable sutures seems to be a safer option in the management of rectal prolapse with reduced mesh-related complications. Total mesh excision should be done whenever it is technically possible.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
FUNDING
The authors received no specific funding for this study.
DATA AVAILABILITY
All data generated or analyzed during this study are included in this published article.



