-
PDF
- Split View
-
Views
-
Cite
Cite
Takanori Aota, Shogo Tanaka, Shigekazu Takemura, Ryosuke Amano, Kenjiro Kimura, Hiroji Shinkawa, Go Ohira, Kohei Nishio, Takeaki Ishizawa, Shoji Kubo, Development of gallbladder cancer during follow-up of pancreaticobiliary maljunction: a report of two cases, Journal of Surgical Case Reports, Volume 2022, Issue 12, December 2022, rjac595, https://doi.org/10.1093/jscr/rjac595
Close - Share Icon Share
Abstract
Pancreaticobiliary maljunction (PBM) is a congenital malformation. The reflux of pancreatic juice into the biliary tract caused by PBM plays a significant role in the development of biliary tract cancers (BTCs), such as gallbladder cancer and cholangiocarcinoma. Previous studies have demonstrated a high incidence of BTC in patients with PBM. However, there are only a few reports of patients who developed BTC after a diagnosis of PBM. We report the cases of two patients who developed gallbladder cancer after being diagnosed with PBM. They had refused treatment and were being managed with follow-up observation alone after the diagnosis of PBM and developed gallbladder cancer after several years of observation. Thus, surgical treatment should be recommended for all patients with PBM in order to prevent the development of BTC. Moreover, long-term, close, regular follow-up is necessary to facilitate the early diagnosis of subsequent BTC in patients with untreated PBM.
INTRODUCTION
Pancreaticobiliary maljunction (PBM) is a congenital malformation in which the pancreatic and bile ducts merge anatomically outside the duodenal wall, causing the pancreatic juice and bile to flow into each other due to the failure of the sphincter of Oddi. PBM is commonly associated with congenital biliary dilatation (CBD). PBM with or without CBD is a risk factor for biliary tract cancer (BTC). The reflux of pancreatic juice into the biliary tract caused by PBM plays a significant role in the development of BTCs, such as gallbladder cancer and cholangiocarcinoma [1–4]. Therefore, cholecystectomy with or without diversion surgery (resection of the extrahepatic bile duct and biliary reconstruction) should be performed immediately after the diagnosis. Patients with PBM have a high incidence of BTC. However, there are only a few reports of patients who developed BTC after a diagnosis of PBM. We report the cases of two patients who developed gallbladder cancer after being diagnosed with PBM.
CASE SERIES
Patient 1
The patient was a 61-year-old woman who was diagnosed with PBM with Type Ic CBD. At 3 years before her presentation, the largest diameter of the common bile duct observed on endoscopic retrograde cholangiopancreatography (a procedure performed if choledocholithiasis is suspected) was 17 mm (Fig. 1a). After the diagnosis, the patient refused treatment and underwent follow-up observation alone. She was asymptomatic and did not visit the hospital regularly. However, at 3 years after the diagnosis of PBM, gallbladder cancer was detected on contrast-enhanced computed tomography (CECT), which was performed as a part of the PBM examination. The patient had a previous medical history of hypertension. Laboratory examinations revealed the following results: C-reactive protein, 2.29 mg/dl; alanine aminotransferase, 43 U/l; gamma-glutamyltransferase, 109 U/l; carcinoembryonic antigen, 4.0 ng/ml and carbohydrate antigen 19–9, 10 U/ml. At her most recent health examination, CECT revealed a 47-mm mass with a contrast effect in the gallbladder and liver invasion despite the absence of a mass 3 years previously (Fig. 1b). The patient then underwent extrahepatic bile duct resection, cholecystectomy, S4a/S5 hepatectomy and regional lymph node dissection. The liver-invading tumor was exposed on the surface, but it did not directly infiltrate into the other organs (Fig. 2a). The amylase level in the bile sample collected from the common bile duct was 136 400 U/l. A pathological examination revealed moderately differentiated adenocarcinoma of the gallbladder without bile duct involvement (T3aN0M0 Stage IIIa) (Fig. 2b). The post-operative course was uneventful, and gemcitabine and cisplatin therapy were administered as adjuvant chemotherapy for 8 months. However, liver and lung metastases developed 8 months after surgery, and the patient died 2 years after surgery.
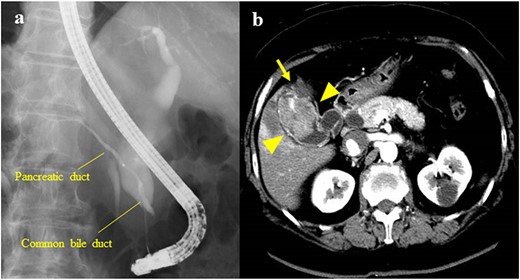
(a) Endoscopic retrograde cholangiopancreatography showing PBM with a Type Ic CBD; the largest diameter of the common bile duct was 17 mm; (b) CECT showing a 47-mm mass with a contrast effect on the gallbladder (arrowhead); the tumor had invaded the S4a/S5 of the liver (arrow).
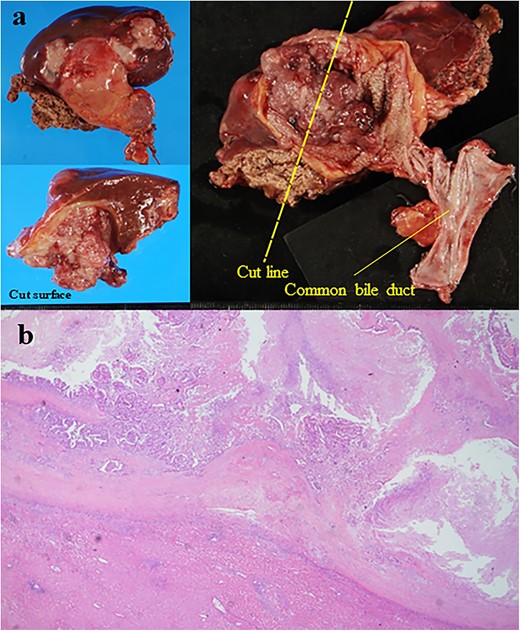
(a) Resected specimen; the liver-invading tumor was observed in the gallbladder (size: 50 × 44 mm); the tumor was exposed on the surface of the liver (b) the histological examination showed moderately differentiated adenocarcinoma (hematoxylin and eosin staining, ×40).
Patient 2
The patient was a 62-year-old man who was diagnosed with PBM with Type Ia CBD 4 years previously (Fig. 3a). On magnetic resonance cholangiopancreatography (MRCP), the largest diameter of the common bile duct was 29 mm. However, the patient refused treatment and underwent follow-up observation alone. He visited the hospital regularly and underwent serum tumor marker tests and imaging (CECT or MRCP) annually despite the absence of symptoms. Approximately, 4 years after his diagnosis, gallbladder cancer was detected on CECT, which was performed as part of the regular follow-up examination for PBM. The patient had a previous medical history of alcoholic cirrhosis (Child–Pugh B), diabetes mellitus and hypertension. Laboratory examinations revealed the following values: platelet count, 9.5 × 104/μl; aspartate transaminase, 47 U/l; gamma-glutamyltransferase, 138 U/l; total bilirubin, 1.4 mg/dl; albumin, 3.4 g/dl; prothrombin time, 65%; carcinoembryonic antigen, 10.4 ng/ml; carbohydrate antigen 19–9, <2 U/ml and duke pancreatic monoclonal antigen type 2, 570 U/ml. In the current examination, contrast-enhanced CT revealed an 11-mm mass with a contrast effect in the gallbladder despite the absence of a mass 4 years previously (Fig. 3b). The patient then underwent extrahepatic bile duct resection and cholecystectomy. The gallbladder had atrophied and had been buried in the liver (Fig. 4a and b). The amylase level in the bile samples collected from the gallbladder and common bile duct were 6590 and 15 270 U/l, respectively. A pathological examination revealed well-differentiated adenocarcinoma of the gall bladder without bile duct involvement (T1aN0M0 Stage I) (Fig. 4c). R0 resection was performed. However, the patient developed hepatic failure, refractory ascites and spontaneous bacterial peritonitis after surgery and died of sepsis after 4 months.
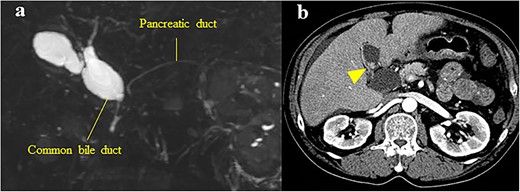
(a) MRCP showing PBM with Type Ia CBD; the largest diameter of the common bile duct was 29 mm; (b) CECT showing an 11-mm mass with a contrast effect in the gallbladder (arrowhead).
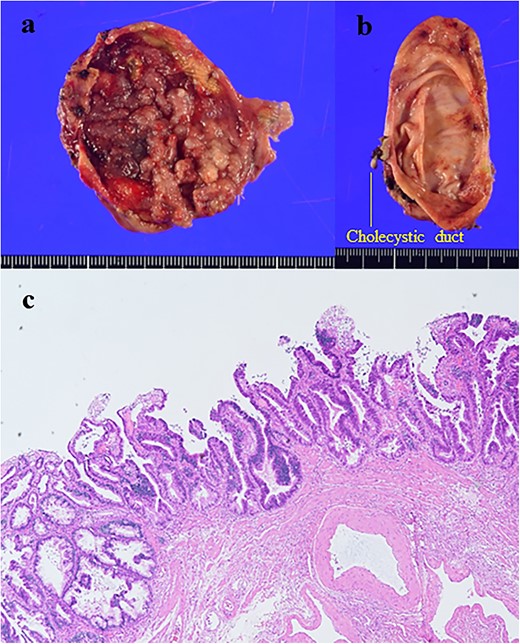
(a) Gallbladder; (b) common bile duct; the tumor was observed in the gallbladder (size: 35 × 30 mm); (c) the histological examination showed well-differentiated adenocarcinoma (hematoxylin and eosin staining, ×100).
DISCUSSION
PBM with or without CBD is a risk factor for BTC. In a Japanese nationwide survey, 270 (21.1%) of 1280 adult patients with CBD and 289 (43.5%) of 665 adult patients with PBM who had no history of biliary dilatation presented with BTC [2]. Only a few patients developed BTC after a diagnosis of PBM. Accordingly, only six reports involving the development of BTC after a diagnosis of PBM were identified in a search of Ichushi-Web and PubMed using the following keywords: ‘pancreaticobiliary maljunction’, ‘congenital biliary dilatation’ or ‘choledochal cyst’ and ‘gallbladder cancer’, ‘cholangiocarcinoma’ or ‘biliary tract cancer’ and ‘follow-up’ [5–10]. These reports, including ours, showed that eight patients without treatment for PBM developed BTC; among these patients, seven patients underwent surgery (Table 1). The staging of the aforementioned cases was as follows: Stage I (n = 5), Stage II (n = 1), Stage IIIa (n = 1) and Stage IV (n = 1). In one of two patients in our series, Stage I gallbladder cancer was detected during a regular follow-up examination for PBM. Such a high proportion of patients with Stage I BTC indicated that BTC developed newly after the diagnosis of PBM and directly showed that PBM is a significant risk factor for BTC. In five of eight patients, BTC was detected >3 years after the diagnosis of PBM. Four of the patients died between 3 months and 2 years after surgery. However, long-term survival was achieved by patients with Stage I BTC. Although cholecystectomy with or without diversion surgery is recommended for PBM with or without CBD, no study has shown the appropriate timing for surgery after the diagnosis of PBM [3, 11, 12]. By contrast, some patients without symptoms, similar to the two patients in this report, insisted on being managed as outpatients with follow-up observation alone even after the diagnosis of PBM. The clinical features and unsatisfactory outcomes of the eight patients (Table 1) indicate that prophylactic surgical treatment should be performed immediately after the diagnosis of PBM in order to prevent the subsequent development of BTC. If surgery is not performed for any reason, regular follow-up should be conducted in patients with untreated PBM to facilitate the early diagnosis of BTC. In addition, long-term follow-up is recommended, considering that some patients with untreated PBM can develop BTC at >3 years after the diagnosis.
| Case . | Year . | Author . | Age . | Sex . | Todani’s classification . | Follow-up period . | Surgical method . | Diagnosis . | Differentiation . | Prognosis . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1992 | Tanaka et al. | 57 | F | Ia | 10 years | Dilated extrahepatic bile duct resection/ cholecystectomy | Cholangiocarcinoma, T1bN0M0 Stage I | Well | Survived 1 year and 2 months after surgery |
| 2 | 1996 | Saito et al. | 59 | F | Unknown | 8 months | Dilated extrahepatic bile duct resection/ cholecystectomy | Gallbladder cancer, T1aN0M0 Stage I | Well | Survived 3 years and 6 months after surgery |
| 3 | 2003 | Fujita et al. | 65 | F | Unknown | 4 years and 9 months | None | Gallbladder cancer, unknown, metastasis of paraaortic LN, Stage IV | Unknown | Died 3 months after diagnosis |
| 4 | 2008 | Nakagawara et al. | 55 | F | Ia | 4 years | Dilated extrahepatic bile duct resection/ cholecystectomy | Cholangiocarcinoma, T1bN0M0 Stage I,invasive | Unknown | Unknown |
| 5 | 2010 | Yara et al. | 79 | M | IVa | 6 months | Dilated extrahepatic bile duct resection/ cholecystectomy | Cholangiocarcinoma, T1aN0M0 Stage I | Moderately | Unknown |
| 6 | 2020 | Yanagaki et al. | 63 | M | IVa | 1 year and 7 months | Subtotal stomach-preserving PD | Cholangiocarcinoma, T2aN0M0 Stage II | Poorly | Died 1 year and 6 months after surgery |
| 7 | 2021 | Our patients | 61 | F | Ic | 3 years | S4a + S5 hepatectomy/ Dilated extrahepatic bile duct resection/ cholecystectomy | Gallbladder cancer, T3aN0M0 Stage IIIa | Moderately | Died 2 years after surgery |
| 8 | 62 | M | Ia | 4 years | Dilated extrahepatic bile duct resection/ cholecystectomy | Gallbladder cancer, T1aN0M0 Stage I | Well | Died 4 months after surgery |
| Case . | Year . | Author . | Age . | Sex . | Todani’s classification . | Follow-up period . | Surgical method . | Diagnosis . | Differentiation . | Prognosis . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1992 | Tanaka et al. | 57 | F | Ia | 10 years | Dilated extrahepatic bile duct resection/ cholecystectomy | Cholangiocarcinoma, T1bN0M0 Stage I | Well | Survived 1 year and 2 months after surgery |
| 2 | 1996 | Saito et al. | 59 | F | Unknown | 8 months | Dilated extrahepatic bile duct resection/ cholecystectomy | Gallbladder cancer, T1aN0M0 Stage I | Well | Survived 3 years and 6 months after surgery |
| 3 | 2003 | Fujita et al. | 65 | F | Unknown | 4 years and 9 months | None | Gallbladder cancer, unknown, metastasis of paraaortic LN, Stage IV | Unknown | Died 3 months after diagnosis |
| 4 | 2008 | Nakagawara et al. | 55 | F | Ia | 4 years | Dilated extrahepatic bile duct resection/ cholecystectomy | Cholangiocarcinoma, T1bN0M0 Stage I,invasive | Unknown | Unknown |
| 5 | 2010 | Yara et al. | 79 | M | IVa | 6 months | Dilated extrahepatic bile duct resection/ cholecystectomy | Cholangiocarcinoma, T1aN0M0 Stage I | Moderately | Unknown |
| 6 | 2020 | Yanagaki et al. | 63 | M | IVa | 1 year and 7 months | Subtotal stomach-preserving PD | Cholangiocarcinoma, T2aN0M0 Stage II | Poorly | Died 1 year and 6 months after surgery |
| 7 | 2021 | Our patients | 61 | F | Ic | 3 years | S4a + S5 hepatectomy/ Dilated extrahepatic bile duct resection/ cholecystectomy | Gallbladder cancer, T3aN0M0 Stage IIIa | Moderately | Died 2 years after surgery |
| 8 | 62 | M | Ia | 4 years | Dilated extrahepatic bile duct resection/ cholecystectomy | Gallbladder cancer, T1aN0M0 Stage I | Well | Died 4 months after surgery |
| Case . | Year . | Author . | Age . | Sex . | Todani’s classification . | Follow-up period . | Surgical method . | Diagnosis . | Differentiation . | Prognosis . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1992 | Tanaka et al. | 57 | F | Ia | 10 years | Dilated extrahepatic bile duct resection/ cholecystectomy | Cholangiocarcinoma, T1bN0M0 Stage I | Well | Survived 1 year and 2 months after surgery |
| 2 | 1996 | Saito et al. | 59 | F | Unknown | 8 months | Dilated extrahepatic bile duct resection/ cholecystectomy | Gallbladder cancer, T1aN0M0 Stage I | Well | Survived 3 years and 6 months after surgery |
| 3 | 2003 | Fujita et al. | 65 | F | Unknown | 4 years and 9 months | None | Gallbladder cancer, unknown, metastasis of paraaortic LN, Stage IV | Unknown | Died 3 months after diagnosis |
| 4 | 2008 | Nakagawara et al. | 55 | F | Ia | 4 years | Dilated extrahepatic bile duct resection/ cholecystectomy | Cholangiocarcinoma, T1bN0M0 Stage I,invasive | Unknown | Unknown |
| 5 | 2010 | Yara et al. | 79 | M | IVa | 6 months | Dilated extrahepatic bile duct resection/ cholecystectomy | Cholangiocarcinoma, T1aN0M0 Stage I | Moderately | Unknown |
| 6 | 2020 | Yanagaki et al. | 63 | M | IVa | 1 year and 7 months | Subtotal stomach-preserving PD | Cholangiocarcinoma, T2aN0M0 Stage II | Poorly | Died 1 year and 6 months after surgery |
| 7 | 2021 | Our patients | 61 | F | Ic | 3 years | S4a + S5 hepatectomy/ Dilated extrahepatic bile duct resection/ cholecystectomy | Gallbladder cancer, T3aN0M0 Stage IIIa | Moderately | Died 2 years after surgery |
| 8 | 62 | M | Ia | 4 years | Dilated extrahepatic bile duct resection/ cholecystectomy | Gallbladder cancer, T1aN0M0 Stage I | Well | Died 4 months after surgery |
| Case . | Year . | Author . | Age . | Sex . | Todani’s classification . | Follow-up period . | Surgical method . | Diagnosis . | Differentiation . | Prognosis . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1992 | Tanaka et al. | 57 | F | Ia | 10 years | Dilated extrahepatic bile duct resection/ cholecystectomy | Cholangiocarcinoma, T1bN0M0 Stage I | Well | Survived 1 year and 2 months after surgery |
| 2 | 1996 | Saito et al. | 59 | F | Unknown | 8 months | Dilated extrahepatic bile duct resection/ cholecystectomy | Gallbladder cancer, T1aN0M0 Stage I | Well | Survived 3 years and 6 months after surgery |
| 3 | 2003 | Fujita et al. | 65 | F | Unknown | 4 years and 9 months | None | Gallbladder cancer, unknown, metastasis of paraaortic LN, Stage IV | Unknown | Died 3 months after diagnosis |
| 4 | 2008 | Nakagawara et al. | 55 | F | Ia | 4 years | Dilated extrahepatic bile duct resection/ cholecystectomy | Cholangiocarcinoma, T1bN0M0 Stage I,invasive | Unknown | Unknown |
| 5 | 2010 | Yara et al. | 79 | M | IVa | 6 months | Dilated extrahepatic bile duct resection/ cholecystectomy | Cholangiocarcinoma, T1aN0M0 Stage I | Moderately | Unknown |
| 6 | 2020 | Yanagaki et al. | 63 | M | IVa | 1 year and 7 months | Subtotal stomach-preserving PD | Cholangiocarcinoma, T2aN0M0 Stage II | Poorly | Died 1 year and 6 months after surgery |
| 7 | 2021 | Our patients | 61 | F | Ic | 3 years | S4a + S5 hepatectomy/ Dilated extrahepatic bile duct resection/ cholecystectomy | Gallbladder cancer, T3aN0M0 Stage IIIa | Moderately | Died 2 years after surgery |
| 8 | 62 | M | Ia | 4 years | Dilated extrahepatic bile duct resection/ cholecystectomy | Gallbladder cancer, T1aN0M0 Stage I | Well | Died 4 months after surgery |
CONCLUSIONS
We presented the cases of two patients who developed gallbladder cancer after a diagnosis of PBM, indicating that PBM is an evident risk factor for BTC. Surgical treatment, such as cholecystectomy with or without diversion surgery, should be performed for patients with PBM in order to prevent the development of BTC. In addition, patients with untreated PBMs require long-term regular close follow-up to facilitate the early diagnosis of subsequent BTC.
ACKNOWLEDGEMENTS
We would like to thank Brian Quinn, Editor-in-Chief, Japan Medical Communication (www.japan-mc.co.jp) for editing the English language of this manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
DATA AVAILABILITY
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
CONSENT FOR PUBLICATION
A written informed consent was obtained for the publication of this case report.



