-
PDF
- Split View
-
Views
-
Cite
Cite
Chiharu Tanaka, Shinichiro Hiraiwa, Hiroyuki Otsuka, Masaomi Yamaguchi, Platelet aggregation with various morphologies of neutrophils in arterial thrombus in a patient with Coronavirus disease: a case report, Journal of Surgical Case Reports, Volume 2022, Issue 11, November 2022, rjac532, https://doi.org/10.1093/jscr/rjac532
Close - Share Icon Share
Abstract
Arterial thromboembolism is a life-threatening condition in COVID-19 patients; however, the mechanism of hypercoagulopathy remains unknown. A 62-year-old man with a history of obesity was diagnosed with COVID-19 pneumonia. After hospitalisation, unfractionated heparin was administered because of increased D-dimer levels; nevertheless, an arterial embolism in the left lower limb developed on the following day. Enhanced computed tomography revealed an occluded left iliac artery and intra-aortic thrombus at the juxtarenal level. Urgent thrombectomy was performed. On post-operative day 6, coumadin was initiated to treat the remaining thrombus. The patient was discharged without any complications. The removed thrombus pathologically presented platelet aggregation and degenerated neutrophils that were in various time axes; some neutrophils had clear margins of nuclear membrane, whereas others had pyknotic and fragment nuclei. We believe that the platelet formation and the neutrophils in several time axes could be key factors in promoting thrombus formation in COVID-19 patients.
INTRODUCTION
Coronavirus disease (COVID-19) is prevalent worldwide, with a large number of patients estimated at over 600 million and over 6.5 million recorded deaths. The hypercoagulopathy state caused by COVID-19 has recently become a major problem [1]; however, the underlying mechanism remains unknown. Here, we report a case of arterial thromboembolism caused by COVID-19 based on histological findings.
CASE REPORT
A 62-year-old man with aggravated symptoms of fever, cough and malaise for 12 days was admitted to the hospital. The patient was obese, with a body mass index of 30.0. The patient was conscious but presented with laboured breathing. His body temperature was 36.7 C, blood pressure was 121/62 mmHg, heart rate was 84/min respiratory rate was 24/min and oxygen saturation was 82% with room air. Non-enhanced computed tomography (CT) showed inflammation in the total lung field (Fig. 1A). Blood test results revealed increased levels of D-dimer of 23.4 mg/L, C-reactive protein of 1600 nmol/L and creatinine of 150.3 μmol/L. The result of SARS-CoV-2 polymerase chain reaction detective kit (TAKARA BIO INC., Shiga, Japan) was positive with N501Y mutation. He was subsequently admitted to the hospital and received oxygen inhalation and intravenous injections of remdesivir, dexamethasone and unfractionated heparin. On the following day, the patient complained of pain and cold sensation in his left leg upon waking. Emergency enhanced CT showed a thrombus in the aorta at the juxtarenal level (Fig. 1B) and an obstructed left iliac artery with a thrombus (Fig. 2A). No cardiac thrombi were observed. The activated partial thromboplastin time was 28 s, and the D-dimer level was unchanged from the previous day, as shown in Fig. 3. Other blood test results showed normal values, including antinuclear antibodies and markers of vasculitis or malignancy.
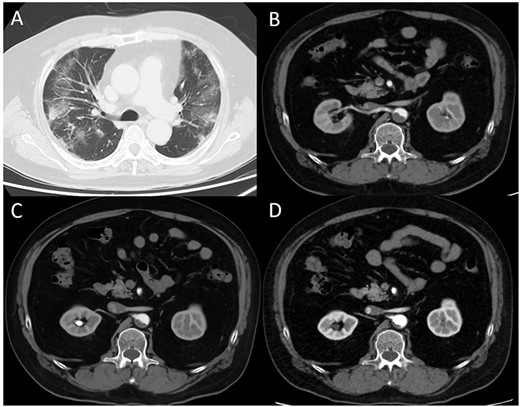
(A) Computed tomography (CT) image obtained on the first day. The bilateral lungs showed inflammation. (B) Enhanced CT image obtained immediately after developing acute limb ischemia. Intra-aortic thrombus was observed at the juxtarenal level. (C) Enhanced CT image obtained on post-operative day 11. The thrombus size had decreased. (D) Enhanced CT image obtained 1 month after surgery. The thrombus had further diminished.
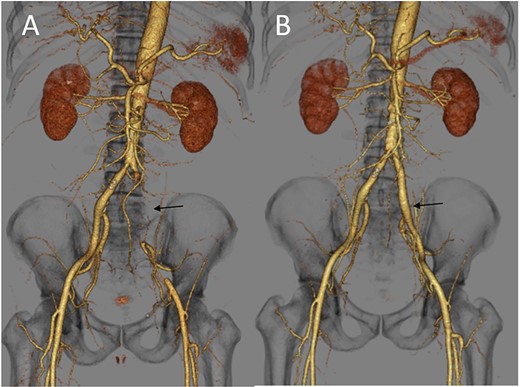
(A) Three-dimensional enhanced CT image obtained immediately after developing acute limb ischemia. The left iliac artery was occluded as indicated by an arrow. (B) Three-dimensional enhanced CT image obtained on post-operative day 11. A patent left iliac artery was observed, as indicated by an arrow.
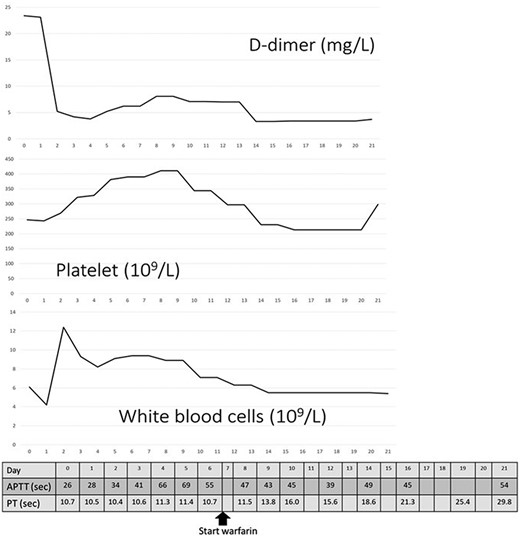
Changes in D-dimer levels, platelet and white blood cell counts, activated partial thromboplastin time and prothrombin time (PT). The horizontal axis refers to days of hospital stay. D-dimer levels had already increased on the day of hospitalisation (day 0) and decreased on day 3, which was after thrombectomy. Platelet counts had not decreased below 200 × 109/L. Unfractionated heparin was administered from day 0 until day 15 when PT was prolonged.
The patient underwent urgent thrombectomy to salvage the left lower limb. Using a Fogarty catheter, fresh thrombus in the left iliac artery and superficial femoral artery was removed. The patient’s hypoxia condition continued until post-operative day 4. From post-operative day 6, coumadin was initiated to treat the remaining thrombus in the aorta (Fig. 1C). After confirming a negative test result for COVID-19, the patient was discharged on post-operative day 20. A month after the surgery, repeat enhanced CT showed a decrease in the quantity of intra-aortic thrombus (Fig. 1D) and the patent left iliac artery (Fig. 2B).
Histological findings of removed thrombus in the iliac artery showed large platelet aggregate formation (CD61 positive by immunohistochemistry) and degenerated inflammatory cells with background meshed fibrin deposition (Fig. 4A and B). The area of aggregated inflammatory cells was distinguished from the acellular pinkish area, which showed spatial stratification (Fig. 4C). The majority of inflammatory cells were neutrophils, which were composed of those in various time axes; some had clear margins of nuclear membrane accompanied by neutrophils, whereas others had pyknotic and fragmented nuclei (Fig. 4D and E). Additionally, the thrombus obtained by the other case of iliac artery embolisation because of chronical atrial fibrillation in an 83-year-old man was examined for comparing. The histological finding showed many inflammatory cells distributed diffusely (Fig. 4F).
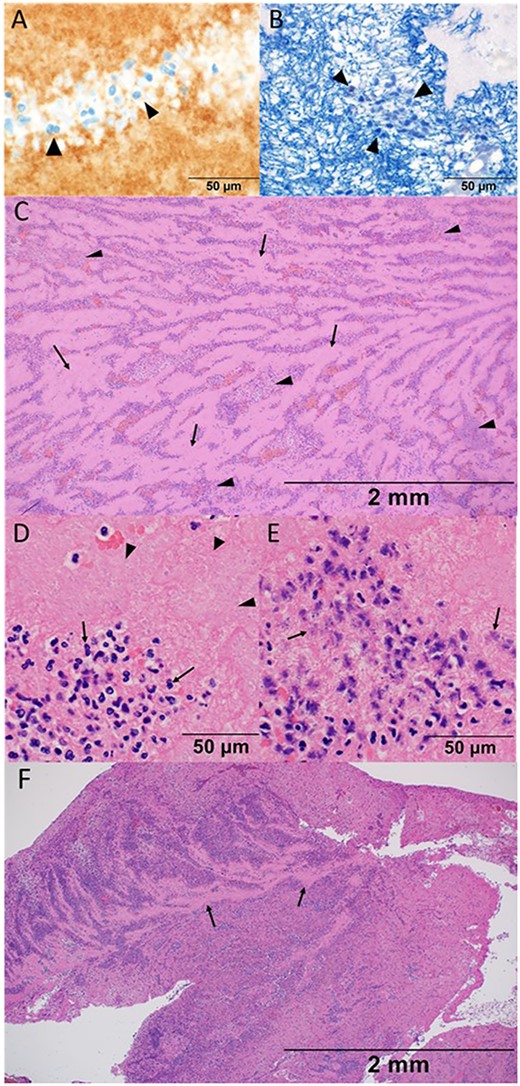
Histological findings of thrombosis removed from the iliac artery in the present case (A–E), and in the other case without COVID-19 infection (F). (A) High-power view with a 50-μm measurement showing the platelet that was confirmed by the morphological feature with eosinophilic fragments as well as CD61 staining as indicated by arrow heads. (B) High-power view with a 50-μm measurement showing fibrin which was stained blue with phosphotungstic acid-hematoxylin stain. Fibrin network was constituted around the nuclei of the inflammatory cells, which was indicated by arrow heads. (C) Low-power view of hematoxylin and eosin stain with a 2-mm measurement showing the histological structure. Acellular pinkish area (arrows) was mildly distinguished with an inflammatory-cell aggregated area (arrow heads). (D) High-power view of hematoxylin and eosin stain with a 50-μm measurement showing the aggregation of neutrophils (arrows) and platelets (arrow heads). Note the clear margin of the nuclear membrane accompanied by neutrophils. (E) High-power view of hematoxylin and eosin stain with a 50-μm measurement. Note and compare the morphological features of neutrophils between (D) and (E). Pyknotic and fragment nuclei of neutrophils were observed (arrows). The elapsed time of the thrombus formation was difficult to assess based on the morphological features, either in the chronic or very acute phase. (F) Low-power view presenting many inflammatory cells that distributed diffusely in the non-COVID-19 case. Acellular area was recognised partially, as indicated by arrows, and did not compose stratification with inflammatory cells.
DISCUSSION
The frequency of arterial thromboembolism caused by COVID-19 is 3.9% [2], and arterial and venous thromboembolisms caused by COVID-19 account for 35% of patients in the intensive care cohort [1]. Arterial thromboembolism has a higher risk of death [3] or limb amputation, which accounts for 25% of cases even in a young population [4]. D-dimer levels predict thrombotic complications and severe conditions [4, 5]. Low-molecular weight heparin as prophylactics for thrombus has decreased the mortality in the patients with D-dimer exceeding 3.0 mg/L [5], and is considered more beneficial for patients with risk factors such as male sex, age > 50 years, history of smoking and obesity, as suggested by Wickham et al. [6]. In our case, the patient presented to the hospital after a 12-day duration of respiratory symptoms. Initial blood tests showed an elevated D-dimer level (Fig. 3), suggesting that a thrombus existed on the day before the development of the arterial occlusion. Additionally, the presence of melting neutrophils indicated that the thrombus was not fresh. Heparin could have prevented thromboembolism; however, this would have been dependent on the quantity of thrombus in the aorta.
Intra-aortic thrombus formation in COVID-19 patients has been previously reported [3, 6]. Regarding Virchow’s sign, unusual hypercoagulopathy played an important role in thrombus formation in the aorta, which was not significantly affected by atherosclerosis in our case. Heparin infusion contributed to better survival rates [3]. In our case, the intra-aortic thrombus decreased 1 month after the surgery, and limb amputation was avoided following the administration of unfractionated heparin and coumadin.
Compared with the histological finding of the thrombus in the other case without COVID-19 infection, the stratum constituted by the acellular area and the neutrophils-aggregated area was more obvious in the present case (Fig. 4C and F). It suggested the platelets agglutinated intensively in the present case; however, serous platelets had not been consumed. Neutrophils were mainly observed as inflammatory cells despite the viral infection, and they were with spatially stratification like stratum in various time axes, which was surprisingly identified in the intra-aortic thrombus.
An autopsy examination showed that 60% of COVID-19 patients had microthrombi in the lungs, heart, kidneys and liver [7]. Satturwar et al. reviewed that the thrombosis was found in almost all organs of COVID-19 patients, and the thrombi comprised of fibrin and/or platelet were found in all sized vessels of the lungs. The etiology of thrombus formation is multifactorial including microvascular dysfunction, immunological response and endothelial injury reported in COVID-19 cases [8]. Some reports also have argued that COVID-19 damages vascular endothelial cells [5, 9].
Satturwar et al. also mentioned the presence of neutrophilic plugs trapped in various organs [8]. Neutrophil extracellular traps (NETs) are considered to be formed by damage to endothelial cells, causing organ damage and contributing to mortality in COVID-19 patients [10, 11]. The etiology of aortic thrombus is considered to be different from that of microthrombus; however, our case findings support the mechanism of thrombosis formation by activated NETs in COVID-19 cases. Therefore, inhibiting NETs’ formation could be key factors in avoiding arterial embolism in COVID-19 patients from a histological point of view. The means to prevent thrombus formation cannot be based on a single case; therefore, further confirmation is mandatory.
CONFLICT OF INTEREST STATEMENT
All authors have no conflict of interest.
FUNDING
None.



