-
PDF
- Split View
-
Views
-
Cite
Cite
Jef Van den Eynde, Roel L F van der Palen, Regina Bökenkamp, Mark G Hazekamp, Delayed biventricular repair of interrupted aortic arch with left ventricular outflow tract obstruction in 22q11.2 deletion syndrome: a case report, Journal of Surgical Case Reports, Volume 2022, Issue 11, November 2022, rjac495, https://doi.org/10.1093/jscr/rjac495
Close - Share Icon Share
Abstract
In patients with critical left ventricular outflow tract obstruction but adequately sized ventricles, the treatment of choice is biventricular repair. Several options have been proposed, including neonatal Yasui or Ross–Konno operation. However, each of these procedures carries a high mortality risk, especially in syndromic neonates. Here, we report the case of a patient with 22q11.2 deletion syndrome and a diagnosis of interrupted aortic arch type B2, ventricular septal defect and left ventricular outflow tract obstruction. As a means to avoid high-risk neonatal surgery in this patient, we pursued a strategy of delayed biventricular repair involving initial hybrid Norwood palliation followed by a Yasui-type operation at 3 months. Although this strategy turned out to be successful, proactive monitoring for the development of ductal stent stenosis during follow-up after the hybrid procedure remains crucial to prevent hemodynamic complications such as cardiac failure and systemic hypoperfusion.
INTRODUCTION
Interrupted aortic arch (IAA) is a rare condition accounting for 1.5% of congenital heart defects (CHD; [1]). In 73% of cases an associated ventricular septal defect (VSD) with posterior displacement of the conal septum is found, resulting in varying degrees of left ventricular outflow tract obstruction (LVOTO; [2]). Since the mitral valve and LV of these patients are adequately sized, the treatment of choice remains biventricular repair, even in critical LVOTO. In the latter scenario, several strategies have been proposed to either replace (Ross–Konno procedure) or augment (Yasui procedure) the LVOT to make it suitable for systemic outflow [3, 4]. However, early mortality rates after neonatal Ross procedure may be as high as 38%, especially when associated with arch repair [5]. Furthermore, although a two-stage Yasui operation (Norwood–Rastelli) has been proposed to reduce morbidity and mortality during the neonatal period, high interstage mortality ~54% has been reported among patients with genetic syndromes [6, 7]. Since genetic syndromes, most commonly 22q11.2 deletion syndrome, occur in 50–70% of patients with IAA/LVOTO, neither of the above approaches seems an ideal option in a sizable part of this patient population.
CASE REPORT
A female newborn, delivered at 39 weeks and 3 days’ gestational age (birth weight 3.4 kg), was admitted to the neonatal intensive care unit (NICU) of our tertiary referral center for planned postnatal care (Table 1). Amniocentesis had revealed 22q11.2 microdeletion and fetal echocardiography allowed for prenatal diagnosis of IAA type B and large inlet VSD with extension to the outlet and posterior displacement of the conal septum resulting in LVOTO. Prostaglandin E1 infusion was immediately instituted. Echocardiography at this point revealed a small, oval shaped LVOT with dimensions 5.0 × 3.7 mm (Fig. 1A and B). The VSD measured 9 mm, whereas the diameters of the aortic valve annulus, sinus of Valsalva and sinotubular junction were 5.2 mm (Z-score − 3.1), 7.6 mm (Z-score − 2.5) and 6.3 mm (Z-score − 1.7), respectively [8]. In addition, the diagnosis of an aberrant right subclavian artery (arteria lusoria) was made, thus indicating IAA type B2.
| Age . | Event . |
|---|---|
| 22 weeks’ gestational age | Prenatal diagnosis of interrupted aortic arch type B, ventricular septal defect and left ventricular outflow tract obstruction |
| 0 days | Birth at 39 weeks and 3 days’ gestation, birth weight 3.4 kg; institution of prostaglandin E1 infusion and admission to the neonatal intensive care unit |
| 1 day | Bilateral pulmonary artery banding for hemodynamic stabilization because of persistent tachypnea, oliguria and elevated lactate |
| 12 days | Ductal stenting to complete the initial hybrid palliation |
| 23 days | Discharge home after first hospitalization |
| 1.7 months | Emergency admission to intensive care unit and repeat catheterization because of restenosis of the ductal stent |
| 2 months | Transfer from intensive care unit to pediatric cardiology ward |
| 2.1 months | Readmission to intensive care unit because of deteriorating left ventricular systolic function despite patent ductal stent |
| 3 months | Yasui-type operation |
| 4.3 months | Transfer from intensive care unit |
| 4.7 months | Discharge home |
| 6.1 months | Doing clinically well with a small residual ventricular septal defect but good biventricular systolic function |
| Age . | Event . |
|---|---|
| 22 weeks’ gestational age | Prenatal diagnosis of interrupted aortic arch type B, ventricular septal defect and left ventricular outflow tract obstruction |
| 0 days | Birth at 39 weeks and 3 days’ gestation, birth weight 3.4 kg; institution of prostaglandin E1 infusion and admission to the neonatal intensive care unit |
| 1 day | Bilateral pulmonary artery banding for hemodynamic stabilization because of persistent tachypnea, oliguria and elevated lactate |
| 12 days | Ductal stenting to complete the initial hybrid palliation |
| 23 days | Discharge home after first hospitalization |
| 1.7 months | Emergency admission to intensive care unit and repeat catheterization because of restenosis of the ductal stent |
| 2 months | Transfer from intensive care unit to pediatric cardiology ward |
| 2.1 months | Readmission to intensive care unit because of deteriorating left ventricular systolic function despite patent ductal stent |
| 3 months | Yasui-type operation |
| 4.3 months | Transfer from intensive care unit |
| 4.7 months | Discharge home |
| 6.1 months | Doing clinically well with a small residual ventricular septal defect but good biventricular systolic function |
| Age . | Event . |
|---|---|
| 22 weeks’ gestational age | Prenatal diagnosis of interrupted aortic arch type B, ventricular septal defect and left ventricular outflow tract obstruction |
| 0 days | Birth at 39 weeks and 3 days’ gestation, birth weight 3.4 kg; institution of prostaglandin E1 infusion and admission to the neonatal intensive care unit |
| 1 day | Bilateral pulmonary artery banding for hemodynamic stabilization because of persistent tachypnea, oliguria and elevated lactate |
| 12 days | Ductal stenting to complete the initial hybrid palliation |
| 23 days | Discharge home after first hospitalization |
| 1.7 months | Emergency admission to intensive care unit and repeat catheterization because of restenosis of the ductal stent |
| 2 months | Transfer from intensive care unit to pediatric cardiology ward |
| 2.1 months | Readmission to intensive care unit because of deteriorating left ventricular systolic function despite patent ductal stent |
| 3 months | Yasui-type operation |
| 4.3 months | Transfer from intensive care unit |
| 4.7 months | Discharge home |
| 6.1 months | Doing clinically well with a small residual ventricular septal defect but good biventricular systolic function |
| Age . | Event . |
|---|---|
| 22 weeks’ gestational age | Prenatal diagnosis of interrupted aortic arch type B, ventricular septal defect and left ventricular outflow tract obstruction |
| 0 days | Birth at 39 weeks and 3 days’ gestation, birth weight 3.4 kg; institution of prostaglandin E1 infusion and admission to the neonatal intensive care unit |
| 1 day | Bilateral pulmonary artery banding for hemodynamic stabilization because of persistent tachypnea, oliguria and elevated lactate |
| 12 days | Ductal stenting to complete the initial hybrid palliation |
| 23 days | Discharge home after first hospitalization |
| 1.7 months | Emergency admission to intensive care unit and repeat catheterization because of restenosis of the ductal stent |
| 2 months | Transfer from intensive care unit to pediatric cardiology ward |
| 2.1 months | Readmission to intensive care unit because of deteriorating left ventricular systolic function despite patent ductal stent |
| 3 months | Yasui-type operation |
| 4.3 months | Transfer from intensive care unit |
| 4.7 months | Discharge home |
| 6.1 months | Doing clinically well with a small residual ventricular septal defect but good biventricular systolic function |
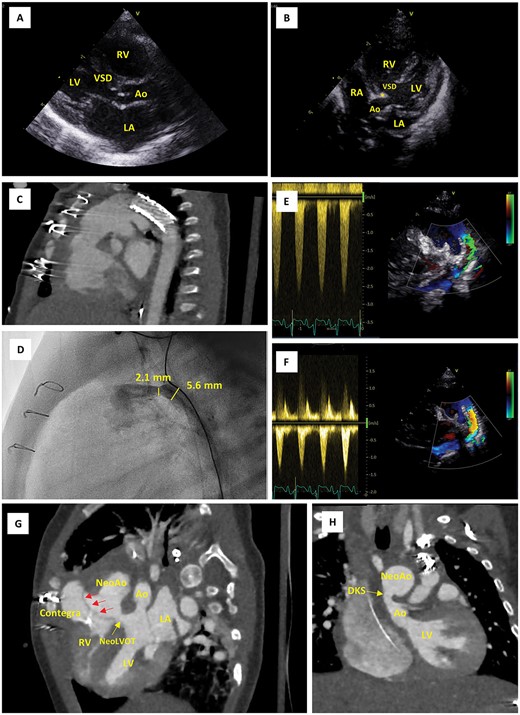
Imaging findings. (A) Parasternal long-axis echo view on postnatal Day 0, showing the large VSD and narrow native LVOT (5.0 mm in this view). (B) Apical five chamber echo view on postnatal Day 0, showing posterior displacement of the conal septum (*) and the narrow native LVOT (3.7 mm in this view). (C) CTA scans after first cardiac catheterization at the age of 12 days, showing position of the ductal stent. (D) Emergency cardiac catheterization at the age of 2 months, showing restenosis of the (non-stented part of the) proximal duct (30 mmHg, diameter 2.1 mm). (E, F) Pulsed wave Doppler evaluation of ductal flow, showing flow acceleration up to 2.5 m/s when the patient was stabilized at the PICU prior to emergency cardiac catheterization (E) and reduction of this flow acceleration to <1.5 m/s following resolution of the stenosis using a Formula 535 vascular balloon-expandable stent (F). (G, H) CTA scan after Yasui procedure; modified views to demonstrate the Rastelli tunnel (red arrows) and the narrow native aortic valve (Ao) (G) as well as the DKS anastomosis (H). Ao, aorta; CTA, computerized tomography angiography; DKS, Damus–Kaye–Stansel; LA, left atrium; LV, left ventricle; PICU, pediatric intensive care unit; NeoAo, neo-aorta; RA, right atrium; RV, right ventricle; VSD, ventricular septal defect.
At the NICU, the neonate became progressively tachypneic, tachycardic and oliguric, whereas saturations remained >90%. Lactate had increased to 4.6 mmol/l. This manifestation resulted from the underlying anatomy (narrow aorta, large VSD and large pulmonary artery) along with an early postnatal decrease in pulmonary vascular resistance, leading to preferential left-to-right shunt through the VSD and arterial duct. For initial non-invasive relief, the neonate was started on continuous positive airway pressure (CPAP) and intravenous furosemide. However, because of refractory cardiac failure the decision was made to proceed with bilateral pulmonary artery banding (PAB) at postnatal Day 1 to optimize systemic perfusion and reduce pulmonary overflow. This procedure was uneventful, showing adequate PAB with right and left pulmonary artery maximum velocities of 3.2 and 3.8 m/s with sawtooth pattern. Hemodynamic stabilization occurred as a result, and no complications followed.
During a multidisciplinary meeting the decision was made to pursue initial hybrid palliation consisting of PAB and ductal stenting, followed by delayed biventricular repair using a Yasui-type operation. This strategy would avoid high-risk neonatal surgery and in the meantime allow for potential growth of the LVOT. Ductal stenting was completed at the age of 12 days. A sinus-SuperFlex-DS stent was positioned in the arterial duct using a soft coronary wire (Fig. 1C). The procedure was uncomplicated and the patient could be discharged home at the age of 23 days.
At 2 months of age, the patient was re-admitted at the emergency department with increased crying, pallor, vomiting and diarrhea. The child was noted to have tachypnea, tachycardia, hepatomegaly by 3–4 cm below the costal margin and dry mucosae. Saturation was 95% at admission but later decreased to 86%. The NT proBNP level was 33 000 pg/ml. Echocardiography revealed a poor systolic right ventricular (RV) and LV function and stenotic flow through the ductal stent. The patient was admitted to the pediatric ICU (PICU) for support with fluids, milrinone, adrenaline and mechanical ventilation, and was subsequently prepared for cardiac cathetherization. In the cathlab, restenosis of the proximal duct (30 mmHg, diameter 2.1 mm) was identified and resolved with a Formula 535 vascular balloon-expandable stent (Fig. 1D–F). The immediate postinterventional course was favorable, although NT proBNP initially remained high and LV function was still moderate. After 10 days, the patient could be transferred from the PICU to the pediatric cardiology ward.
On the third day at the pediatric cardiology ward, the patient had an episode of increased tachycardia, diaphoresis and pronounced desaturation (as low as 79–81%). Echocardiography showed deterioration of LV systolic function but confirmed patency of the ductal stent. The patient was again admitted to the PICU to initiate milrinone, digoxin, bisoprolol and continuous tube feeding. The clinical condition and ventricular systolic function gradually recovered. The remainder of the PICU stay was notable for a Streptococcus hominis bacteremia, which was treated using vancomycin and ceftazidime. The patient remained in the PICU supported on milrinone, digoxin and bisoprolol for 31 days.
At the age of 3 months, while still in the PICU, the patient underwent a Yasui-type procedure to complete the two-stage biventricular repair (Fig. 1G–H). The procedure was performed via median sternotomy with cardiopulmonary bypass, using the brachiocephalic artery and the duct for arterial cannulation and the right atrium and inferior vena cava for venous cannulation. The duct, arch and arch arteries were dissected free, taking care not to injure the left laryngeal recurrent nerve. Following clamping of the ascending aorta and administration of anterograde cardioplegia, a right atriotomy was performed. The left heart was decompressed through the atrial septal defect (ASD) and the arterial cannula was removed from the ductus. The ductal stent was then removed and the ductus was ligated at the pulmonary side. The pulmonary artery bifurcation was detached from the pulmonary trunk. The patient was cooled to 19°C and anterograde cerebral perfusion was initiated to protect the brain during arch repair. The descending aorta was anastomosed end-to-side to the left carotid artery and distal ascending aorta. A Damus–Kaye–Stansel (DKS) anastomosis was then performed. Arch repair was completed by anastomosing the pulmonary trunk and DKS to the remainder of the arch. Both subclavian arteries were sacrificed using clips without connecting them to the aorta. Although perfusion to the body was restored and the patient was gradually rewarmed, the distal anastomosis of a 12-mm Contegra conduit to the pulmonary artery bifurcation was created. A right ventriculotomy was made and the VSD was closed in a fashion similar to the Rastelli procedure, such that the LV would connect to the pulmonary and aortic valves. Through the right atriotomy, the anteroseptal commissure of the tricuspid valve was closed, thereby achieving only trivial residual tricuspid regurgitation, and the ASD was closed. Finally, the proximal anastomosis of the Contegra conduit was completed and the right atriotomy was closed. Then cardiopulmonary bypass was discontinued, hemostasis was achieved, pacemaker leads and drains were left in place, and the sternotomy was covered with a sterile plastic foil to pursue delayed sternal closure on postoperative Day 3.
The patient had an uneventful early postoperative course and demonstrated good biventricular systolic function. The later postoperative course was notable for a hospital-acquired pneumonia and Serratia liquefaciens bacteremia, which were both treated with cotrimoxazole, a short episode of delirium for which risperidone was administered, and three episodes of convulsions. The multiple episodes of infection in the patient’s postoperative course were attributed to thymus agenesis, which is related to 22q11.2 deletion syndrome and had been confirmed at the time of surgery. The convulsions were all lateralized, involved myoclonus of the arm and leg along with nystagmus, and lasted <1 min; all were controlled with midazolam and levetiracetam. No abnormalities on brain ultrasound were noted. In agreement with neurology, levetiracetam was initiated as maintenance therapy for a duration of 3 months. The convulsions eventually did not recur, although residual arm paresis and hemi-attention/hemi-anopsia were noted after the last episode. The patient was extubated 9 days after the procedure and transferred to the pediatric cardiology ward after 38 days. The patient could be discharged home 11 days later and is currently doing clinically well at 6.1 months of age with a small residual VSD (gradient 60 mmHg) but good biventricular systolic function. She has had no convulsions on follow-up and her the arm paresis and hemi-attention/hemi-anopsia have resolved. Her neurological development is reassuring.
DISCUSSION
In this case report, we described a patient with 22q11.2 deletion syndrome and a diagnosis of IAA type B, VSD and LVOTO. This particular combination of features would have predisposed the patient to a high-risk operative course in the neonatal phase if conventional strategies would have been attempted. Given these considerations, we decided instead to pursue a strategy involving initial hybrid palliation followed by a Yasui-type operation at 3 months of age. The results in this patient demonstrate that in this setting, delayed biventricular repair can successfully be achieved. Nonetheless, proactive monitoring for the development of ductal stent stenosis during follow-up after the hybrid procedure remains crucial to prevent hemodynamic complications such as cardiac failure and systemic hypoperfusion.
DATA AVAILABILITY
All data have been provided within the manuscript.
CONFLICT OF INTERESTS STATEMENT
None declared.
FUNDING
None declared.
CONSENT FOR PUBLICATION
The parents have consented to the submission of the case report to the journal.
CONSENT TO PARTICIPATE
Informed consent was obtained from the parents.



