-
PDF
- Split View
-
Views
-
Cite
Cite
Ateeqa Mujeeb Ullah, Anna Jaysing, Hassan Raza Hashmi, Amir Humza Sohail, Wendi Li, John D Allendorf, Suparna A Sarkar, Primary small bowel adenocarcinoma with loss of nuclear expression of PMS2 after resection of mucinous cholangiocarcinoma, Journal of Surgical Case Reports, Volume 2022, Issue 1, January 2022, rjab628, https://doi.org/10.1093/jscr/rjab628
Close - Share Icon Share
Abstract
Mucinous cholangiocarcinoma is an extremely rare form of intrahepatic cholangiocarcinoma that has been characterized by rapid growth, widespread metastasis and poor prognosis. These tumors have been shown to be a part of the Lynch syndrome tumor spectrum, however, the role of DNA mismatch repair (MMR) deficiency in their development is poorly understood. We present the case of a 74-year-old male with cholangiocarcinoma, who underwent Roux-en-Y hepaticojejunostomy and extended left hepatectomy and was diagnosed with a primary small bowel adenocarcinoma 2 years later. Immunohistochemistry testing for mismatch repair proteins was significant for the loss of nuclear expression of PMS2. Taken together, the cause of both the mucinous cholangiocarcinoma and primary small bowel adenocarcinoma with PMS2 loss in the patient presented here is likely genetic, suggestive of a cancer syndrome.
INTRODUCTION
Mucinous cholangiocarcinoma is an extremely rare form of intrahepatic cholangiocarcinoma that has been characterized by rapid growth, widespread metastasis and poor prognosis [1]. These tumors have been shown to be a part of the Lynch syndrome tumor spectrum, however, the role of DNA mismatch repair (MMR) deficiency in their development is still under research [2]. This report presents a case of a 74-year-old male with a history of cholangiocarcinoma, who underwent Roux-en-Y hepaticojejunostomy and extended left hepatectomy and was diagnosed with a primary small bowel adenocarcinoma with loss of PMS2, 2 years later.
CASE REPORT
We present a case of a 74-year-old male who presented in 2018 with abdominal pain and underwent a series of cross-sectional imaging (CT-scan) which revealed an obstructed left bile duct and an associated mass. Follow-up esophagogastroduodenoscopy (EGD) and endoscopic ultrasound (EUS) confirmed the presence of a mass at the confluence of the bile ducts with possible liver invasion (Fig. 1A). A needle aspiration done was suspicious for carcinoma. Patient underwent diagnostic laparoscopy which revealed no evidence of metastatic disease, therefore, an extended left hepatectomy with a roux-en-y hepaticojejunostomy was performed. On pathological evaluation of the specimen, invasive mucinous adenocarcinoma with an intraductal papillary mucinous component, in a background of high-grade dysplasia was found involving the left hepatic lobe (Segment 4b) and left hepatic duct (Fig. 1B). Surgical margins were negative for carcinoma. No lymphovascular, perineural or lymph node involvement was seen. The tumor was classified as pT1a, pNx R0 according to the American Joint Committee of Cancer (AJCC) 8th edition.
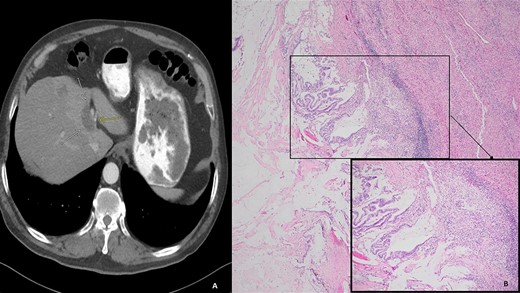
(A) Computed tomography (axial): ovoid low-density mass contiguous with dilated left hepatic bile duct. (B) Histology: invasive mucinous adenocarcinoma with an intraductal papillary mucinous component, in a background of high-grade dysplasia was identified involving the left hepatic lobe (segment 4b) and left hepatic duct.
Two years later, the patient presented again with abdominal pain and rectal bleeding. On CT scan of abdomen and pelvis a lobular circumferential enhancing mass of the proximal fourth portion of the duodenum, with shotty adjacent lymph nodes was found, that was suspicious for primary duodenal adenocarcinoma. As part of diagnostic workup, the patient underwent an EGD (Fig. 2). The biopsy confirmed the presence of invasive moderately differentiated adenocarcinoma and immunohistochemistry testing for mismatch repair proteins (MMR) was significant for the loss of nuclear expression of PMS2 (Fig. 3). The patient underwent resection of the small bowel between the ligament of Treitz and the previous jejunojejunostomy site and end-to-side duodenojejunostomy was performed between the end of the fourth portion of the duodenum and the side of the distal limb of the Roux-en-Y portion of bowel that had previously been created for the hepaticojejunostomy, to reestablish intestinal continuity.
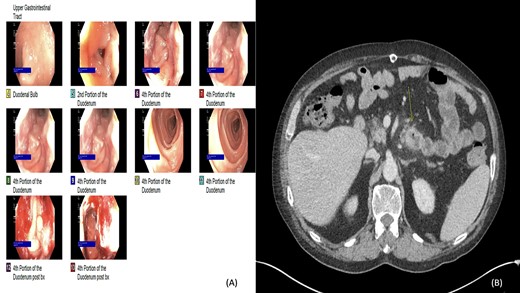
(A and B) Esophagogastroduodenoscopy (EGD) and computed tomography: lobular circumferential enhancing mass of the proximal fourth portion of the duodenum.
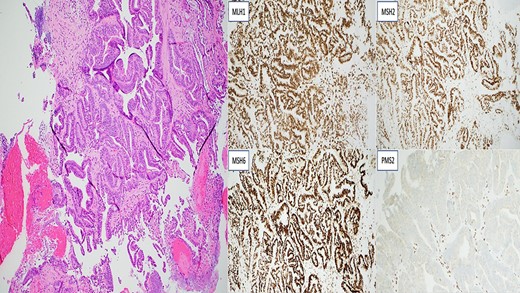
Invasive moderately differentiated adenocarcinoma with immunohistochemistry testing for mismatch repair proteins (MMR) significant for the loss of nuclear expression of PMS2 with retained strong expression of MSH2, MSH6 and MLH1.
Post resection, surgical pathology specimen revealed a 5.8 cm invasive moderately differentiated adenocarcinoma with mucinous features invading the mesenteric fat of the small bowel without lymphovascular or perineural invasion. Surgical margins were negative for carcinoma. Remarkably, 1 of 25 lymph nodes was associated with acellular mucin. Multiple deeper levels and immunohistochemical stain for CAM5.2 failed to reveal isolated tumor cells. Additionally, in the absence of any neoadjuvant therapy, the acellular mucin-associated lymph node was considered uninvolved by adenocarcinoma, as per AJCC conventions (Fig. 4) A histomorphological comparison of small bowel adenocarcinoma and prior cholangiocarcinoma was made at the intradepartmental consensus conference and the two tumors were deemed as separate primaries. The small bowel adenocarcinoma was staged as pT3N0. Genetic analysis of the tumor by sequencing confirmed the MLH1 gene [K618del] mutation, in addition, a common hereditary gene panel also revealed multiple mutations with lesser frequency. At the time of writing this report, the patient shows no radiological evidence of recurrence.
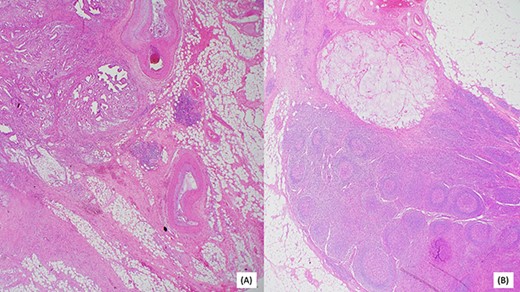
(A) Invasive moderately differentiated adenocarcinoma with mucinous features invading the mesenteric fat of the small bowel. (B) One of 25 lymph nodes associated with acellular mucin.
DISCUSSION
Mucinous cholangiocarcinoma is a rare type of intrahepatic cholangiocarcinoma, characterized by rapid growth, extensive metastasis and poor prognosis [1, 3, 4]. Proposed risk factors include a history of biliary disease, chronic hepatitis C and intrahepatic C. sinensis flukes [5, 6]. In addition, mismatch repair deficiency (MMR-d), which is associated with hereditary cancer syndromes such as Lynch syndrome, has been shown to be associated with mucinous cholangiocarcinoma [2, 7, 8]. However, the patients with MMR-d and mucinous cholangiocarcinoma described by Ju et al. did not have any other typical Lynch syndrome–associated cancers documented. Therefore, it is possible that the first sign of a hereditary cancer syndrome in some patients is mucinous cholangiocarcinoma.
Primary small bowel adenocarcinoma is a similarly a rare gastrointestinal track malignancy characterized by nonspecific symptoms, late diagnosis and poor prognosis [9]. Risk factors include alcohol consumption, as well as Lynch syndrome, the basis of which is a germline MMR mutation [10, 11].
Both somatic and germline mutations in MMR genes lead to the loss of MMR function. Although there are seven known MMR proteins, MLH-1, MSH-2, MSH-6 and PMS-2 are of the most oncologic relevance [12]. The result of a deficiency in any one of these proteins can be variation in the lengths of the microsatellites, termed microsatellite instability (MSI) [13]. Though cases are limited, its suggested that MSI-H cholangiocarcinomas are more likely to be of atypical histomorphology (solid, mucinous or signet-ring) [8]. Similarly, small bowel adenocarcinoma has a high rate of MSI [14].
Taken together, the cause of both the mucinous cholangiocarcinoma and primary small bowel adenocarcinoma with PMS2 loss in the patient presented here is likely genetic, suggestive of a cancer syndrome. A negative history of a hereditary cancer syndrome warrants further evaluation and judicious follow-up and surveillance to prevent recurrence.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
Author notes
A. Mujeeb Ullah and A. Jaysing are both first authors.



