-
PDF
- Split View
-
Views
-
Cite
Cite
Timothy Peacock, Luke Liu, Superior mesenteric artery aneurysm with rupture: an atypical cause of abdominal pain, Journal of Surgical Case Reports, Volume 2022, Issue 1, January 2022, rjab604, https://doi.org/10.1093/jscr/rjab604
Close - Share Icon Share
Abstract
Superior mesenteric artery aneurysm management has evolved in the last 20 years with a greater emphasis on interventional radiological intervention. This case reviews a 60-year-old lady who had a ruptured superior mesenteric aneurysm resulting in a large mesenteric haematoma.
INTRODUCTION
Visceral artery aneurysms (VAAs) are relatively rare, more commonly affecting the splenic and hepatic arteries. Only 10% of cases involve the coeliac or mesenteric vessels. Superior mesenteric artery (SMA) rupture can be associated with significant morbidity from associated bowel ischaemia. It remains an important differential diagnosis for abdominal pain. This case report details the common presentations of VAAs and the management strategies.
CASE REPORT
A 60-year-old lady presented to the emergency department following a syncopal episode with head strike. The syncope was associated with central abdominal pain radiating to the back. Initially, she was haemodynamically normal, but had a transient drop in blood pressure to 75/60, which improved with intravenous fluid resuscitation. The remainder of her observations were normal, with a heart rate of 70 beats per minute in sinus rhythm. She had no significant medical history or cardiovascular risk factors. On examination, her abdomen was soft but tender centrally, with no peritonism. No masses were palpable and the abdominal aorta was not palpable. Initial pathology investigations showed haemoglobin of 7.8 dg/l with an international normalized ration of 1.3.
Initially, the main diagnosis considered was an aortic dissection. Other initial general surgical differentials were a perforated viscous or severe pancreatitis. Computed tomography (CT) angiogram showed a 6 mm pseudoaneurysm arising from a branch vessel 3 cm distal to the origin of the SMA. There was no active arterial blush; however, there was a large associated mesenteric haematoma (measuring 4.5 × 8.0 × 13 cm) with large volume haemoperitoneum (Figs 1–3). There was also a splenic artery aneurysm measuring 5 mm with no evidence of active haemorrhage. After the CT scan, she was noted to be haemodynamically unstable when lying supine, due to inferior vena cava compression by the mesenteric haematoma. A pillow was placed under her left lateral side to act as a wedge to reduce this. She proceeded to urgent angioembolization by interventional radiology, which confirmed the pseudoaneurysm on angiography (Fig. 4, left). The SMA was accessed and the aneurysm was successfully embolized with 3 mm diameter 15 cm length Ruby micro-soft detachable coil (Fig. 4, right).
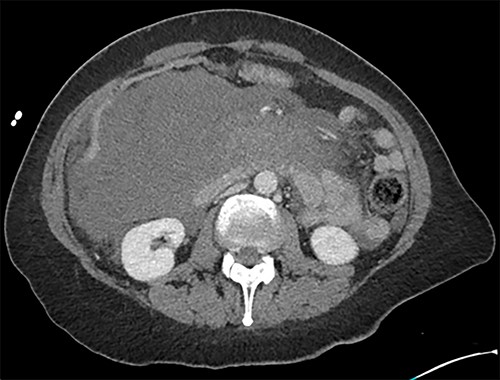
Axial image of the large retroperitoneal haematoma in portal venous phase.
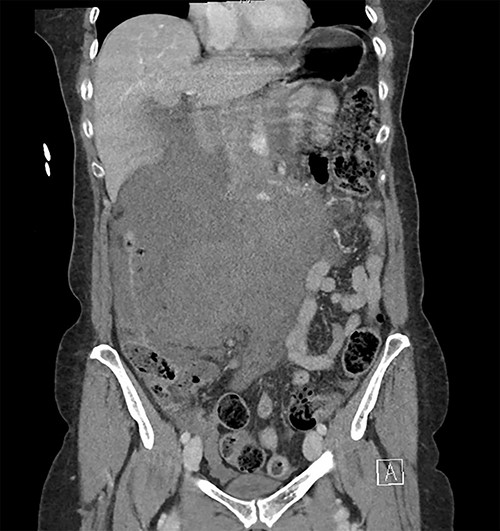
Coronal image of the retroperitoneal haematoma in portal venous phase.
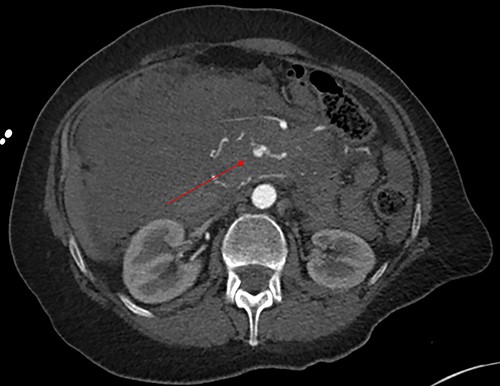
Axial image with arterial phase, demonstrating the pseudoaneurysm arising from a branch of the SMA. There is no active arterial blush.
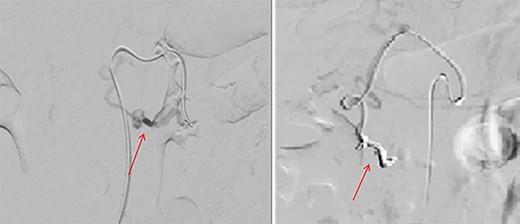
Pre-embolization (left) and post-embolization with coils (right).
On day one after embolization, transthoracic echocardiogram (for workup of syncope) identified normal tricuspid valve morphology, with moderate regurgitation, and mildly elevated right ventricular systolic pressure of 39 mmHg. CT pulmonary angiogram demonstrated segmental bilateral pulmonary embolisms. She was commenced on a heparin infusion and converted to apixaban on discharge. Rheumatological investigation identified no evidence of a systemic vasculitis. CT carotid angiogram and circle of Willis showed normal formation of the cranial and extracranial vessels with no additional aneurysms.
DISCUSSION
SMA aneurysms are rare, accounting for only 10% of VAAs. The more common sites of VAAs are the splanchnic (60%) and hepatic arteries (20%) [1]. Symptoms vary depending on the anatomical location and are often vague, leading to a delay in the diagnosis. Rupture can result in life-threatening bleeding, which requires urgent intervention. SMA aneurysms are generally found incidentally on CT scan. The increased use of CT scans in healthcare has resulted in increased incidental detection of aneurysms [2]. SMA aneurysms may present with vague abdominal pain, nausea, or vomiting. Aneurysm rupture may present with severe abdominal pain with haemodynamic instability and may even progress to abdominal compartment syndrome.
There are multiple risk factors for SMA aneurysms, including atherosclerosis, pregnancy, Marfan disease, Ehlers-Danlos syndrome, fibromuscular dysplasia, Kawasaki’s disease, hereditary haemorrhagic tenlangiectasias and infective endocarditis [3].
Laboratory tests are non-specific, but may demonstrate a haemoglobin drop [4]. A CT mesenteric angiogram is the diagnostic test of choice, which will demonstrate the aneurysm and presence of an active arterial blush. In an elective setting ultrasound can be used for assessment as it gives further information about velocities and blood flow within the aneurysm.
MANAGEMENT
There is no randomized controlled trial that has demonstrated the optimal management for VAAs. Case–control series suggest that early intervention is preferred to close observation. It is presumed that aneurysms with diameter <2.5 cm are unlikely to rupture [5]. Furthermore, superior mesenteric aneurysms are less likely to rupture than hepatic artery aneurysms. However, rupture of an SMA aneurysm exposes patients to significant risk, with one case series demonstrating that three out of eight patients requiring emergent embolization also required a small bowel resection due to bowel ischaemia after embolization. In contrast, elective embolization of SMA aneurysms prior to rupture is associated with low intervention rates for bowel ischemia, with combined case series involving a total of 40 patients having no complications post-procedurally requiring small bowel resection [6, 7]. Therefore, prophylactic elective intervention is generally advocated.
Historically, surgical intervention involved laparotomy with ligation of the aneurysm or bypass in situations where collateral circulation was inadequate [8]. Surgical intervention has largely been surpassed by endovascular intervention, due to the higher rates of morbidity and mortality attached to surgery [9].
Endovascular repair has been widely performed since 2013 and involves gaining access through either the femoral or radial artery. Different embolic agents have been utilized including coils, glue, onyx, Gelfoam, polyvinyl alcohol particles (PVA), and Amplatzer vascular plugs, with the most commonly used embolic agents being coils and PVA [10]. Complications after elective embolization are rare, but patients should be closely monitored in the post-procedural phase. Potential complications include access site complications (including formation of pseudoaneurysm, haematoma and arterial thrombosis), periprocedural rupture of the aneurysm, distal thromboembolism, coil migration, bowel ischaemia and re-bleed requiring further intervention [11, 12].
CONCLUSION
Although SMA aneurysms represent a small proportion of the classical abdominal pain presentations in the emergency department, an understanding of the management strategies is crucial. A multidisciplinary approach with interventional radiology is required, with close monitoring by the general surgical service after intervention to identify potential complications. The mainstay of therapy is interventional embolization given the reduced risk of complications associated with such. Although surgery is an option, it carries significant morbidity risk.
FUNDING
The authors have no financial interest to declare.
AVAILABILITY OF DATA
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
CONSENT
Written consent was obtained by the patient prior to the preparation of this case report.



