-
PDF
- Split View
-
Views
-
Cite
Cite
L M Goroztieta-Rosales, J Gómez-Farías, K D López-García, D O Davila-Rodriguez, Lemmel syndrome: an extraordinary cause of obstructive jaundice—a case report, Journal of Surgical Case Reports, Volume 2022, Issue 1, January 2022, rjab593, https://doi.org/10.1093/jscr/rjab593
Close - Share Icon Share
Abstract
Lemmel syndrome is created by a periampullary duodenal diverticulum. It is identified incidentally in 22% of the population, <10% present with jaundice, pain in the right flank and alteration of bilirubins, transaminases and/or pancreatic enzymes. Its diagnosis and therapeutic management can be carried out successfully with endoscopic retrograde cholangiopancreatography (ERCP), although some cases will require surgical management. We present the case of a 72-year-old male with recurrent obstructive jaundice and suspected choledocholithiasis managed with ERCP, identifying Lemmel syndrome. We recognize the importance of considering this extraordinary cause of obstructive jaundice in order to be able to make a diagnosis and offer timely treatment.
INTRODUCTION
Lemmel syndrome (LS) constitutes the presence of obstructive jaundice in the absence of choledocholithiasis or pancreatic-biliary tumors [1].
It was first reported in 1934 by Lemmel, who described a periampullary duodenal diverticulum (PDD), a potentially obstructive sac-like outlet pouch from the duodenal mucosa.
Most of their presentation is found in asymptomatic patients, and they can be identified incidentally in up to 22% of the population, of which <10% will present with symptoms [2].
Clinically, LS consists of pain in the right upper quadrant (RUQ), and elevated levels of bilirubin, transaminases and/or pancreatic enzymes, consequently from the involvement of the ampulla of Vater [3].
Nowadays, the use of endoscopic treatment using endoscopic retrograde cholangiopancreatography (ERCP) is recommended for the diagnosis of PDD leading to LS [4].
CASE REPORT
Our patient is a 72-year-old male with a history of severe alcoholism for 8 years. Six hours prior to admission, he presented a severe colic type pain located in the epigastric region which radiated to the RUQ. The pain was triggered by the ingestion of cholecystokinetics, associated with nausea and scleral icterus, and managed with antispasmodic with a partial decrease in pain. A previous 9-month history of jaundice lasting 72 h with spontaneous remission was reported.
Physical examination showed scleral icterus and painful abdomen on palpation in the epigastrium and RUQ. Fever, tachycardia, mental status alterations were ruled out. Cardiopulmonary exploration was uneventful. Murphy and Blumberg sign were negative, normal bowel sounds were heard, neither visceromegaly nor tumors were palpated, rest without abnormalities.
Upon admission, laboratories showed a normal hemoglobin (15 g/dl), hematocrit (48%), leucocytes (6700/mm3), platelets (528000), total bilirubin (5.19 mg/dl), direct bilirubin (2.21 mg/dl), alkaline phosphatase (252 U/L), gamma-glutamyl transferase (321 U/L), aspartate aminotransferase (24 U/L), alanine aminotransferase (37 U/L), albumin (3.9 g/dl), prothrombin time 10.3 s, partial thromboplastin time 21.2 s, International Normalized Ratio (1.06), amylase (50 U/L) and lipase (43 U/L).
Due to the obstructive jaundice syndrome, hepatic and biliary system ultrasound was performed, which reported a gallbladder with preserved diameters and a 3 mm wall, 2 mobile 5 mm vesicular stones and a 7 mm common bileduct.
The patient was staged as ‘high risk’ according to the 2019 guidelines of American Society for Gastrointestinal Endoscopy risk classification guidelines for choledocholithiasis. The decision to perform an ERCP was taken, where an obstructive PDD was identified (Fig. 1). An absence of stones or biliary tumors was recognized (Fig. 2B) Oddi’s sphincterotomy and balloon sweep were performed, obtaining a control cholangiography without filling defects (Fig. 2). The initial presumptive diagnosis of choledocholithiasis was ruled out and a diagnosis of LS wasdone.
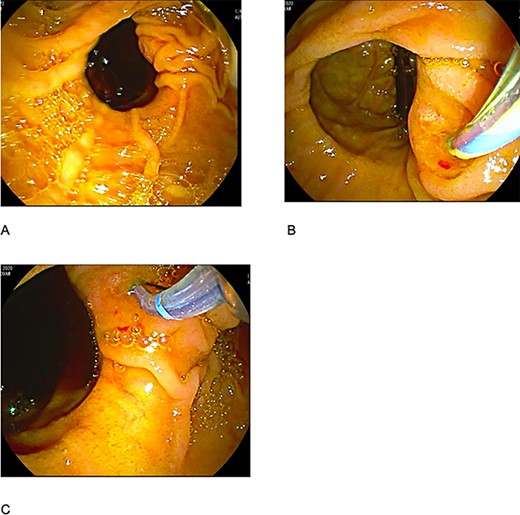
(A) duodenal diverticulum type C of Noda Classification; (B) bile duct deep cannulation; (C) sphincterotomy.
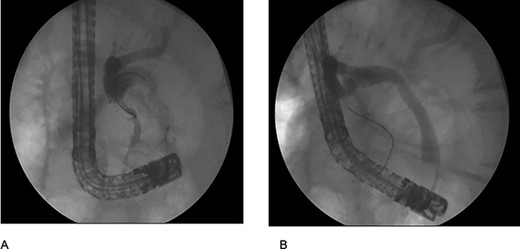
(A) cholangiography shows the bile duct with an extrinsic compression by the duodenal diverticulum witch conditions an angulation; (B) cholangiography after use the retrieval ballon catheter shows absence of stones.
Hepatic function tests were monitored at 24 and 48 h post-ERCP with a decrease in total bilirubin (2.6 mg/dl), direct bilirubin (1.4 mg/dl), alkaline phosphatase (276), gamma-glutamyltransferase (61 U/L), aspartate aminotransferase (42 U/L), alanine aminotransferase (72 U/L).
During hospitalization, he was managed with intravenous analgesics and prophylactic intravenous ceftriaxone, no evidence of systemic inflammatory response was identified, moreover, adequate evolution ensued.
A laparoscopic cholecystectomy was proposed with further refusal by the patient. As of today, the patient has undergone two follow-up visits at 6 months, being found asymptomatic.
DISCUSSION
Of all the types of duodenal diverticulum, the periampullary are the most frequent [5], LS is caused by these with association to obstructive jaundice; patients are usually asymptomatic, although 5% may present with complications such as recurrent gallbladder or bile duct stones, obstructive jaundice, cholangitis or acute pancreatitis, less frequently they present diverticulitis, hemorrhage, perforation or fistula formation [6].
The pathophysiology of LS depends on the location of the diverticulum, chronic fibrosis of the papilla can occur secondary to periampullary diverticulitis and chronic inflammation of the ampulla [7]. As well as a dysfunction of the sphincter of Oddi, or by external compression of the common bile duct or the ampulla of Vater by the PDD [6].
LS can be the source of bile stasis cholangitis. Mild to severe cases of SL-associated cholangitis have been described, as well as infrequent pathogen findings documented by Shinji Miyajima in 2018 [8], with the isolation of the Enterobacteriaceae Edwardsiella dela.
Images are essential for LS diagnosis. Computed tomography is the most performed study in patients in an emergency room scenario, complaining of acute symptoms. Magnetic Resonance Cholangiography or ERCP [9] are indicated, the latter has the advantage of representing an effective mixed modality (diagnostic/therapeutic), which allows for a sphincterotomy and placement of a biliary stent. In high-risk patients, ERCP may represent the best therapeutic option, which is associated with a reduction in the risk of morbidity and mortality [10, 11]. However, it is important to recognize the possibility of technique failure and complications because the papilla is more frequently located in or next to the diverticulum [11].
In case of biliary obstruction, hemorrhage or perforation, diverticulectomy has been described, however it is a complex procedure which has been associated with higher mortality and morbidity [12].
To conclude, we emphasize the importance of making our patient’s case known and the management that we successfully carried out with endoscopy because it was a patient without systemic inflammatory response syndrome or other complications. LS is scarcely reported, although it is a well-described but extraordinary cause of obstructive jaundice. We believe that it should be part of the physician’s competencies to carry out a timely diagnosis and treatment. In the current literature, the endoscopic approach is recognized as the gold standard for diagnosis, it can be used as a therapeutic measure, however, surgical procedures are reserved in complex cases or with associated complications.
CONSENT
Informed consent was obtained for this case report.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
ACKNOWLEDGEMENT
The authors thank the gastrointestinal endoscopy unit, for providing endoscopy images.



