-
PDF
- Split View
-
Views
-
Cite
Cite
Chih-Hsun Lin, Kai Hsia, Jen-Her Lu, Hsu Ma, Cryopreserved allogenic vascular graft in free-flap reconstructive microsurgery: case report, Journal of Surgical Case Reports, Volume 2021, Issue 8, August 2021, rjab375, https://doi.org/10.1093/jscr/rjab375
Close - Share Icon Share
Abstract
The use of cryopreserved allogenic vascular graft in reconstructive microsurgery has rarely been reported. Here, we report a case of lower extremity reconstruction using cryopreserved hepatic artery as the vein conduit. Postoperative flap perfusion was uneventful with satisfactory wound healing, and graft patency was observed on follow-up color Doppler. Thus, cryopreserved allogenic vascular graft could be a source of vascular conduit in microsurgery.
INTRODUCTION
Vascular conduit provides a bridge between the flap pedicle and recipient vessels in free-flap reconstructive microsurgery, when the distance between the wound and recipient vessels is longer than the pedicle length. This situation usually occurs in challenging cases with prior neck dissection, prior free-flap radiotherapy or trauma [1–4].
Autologous vein graft is most commonly used as a vascular conduit in microsurgery, including the greater saphenous vein, cephalic vein, dorsal foot vein, external jugular vein or intra-flap vein [5]. Use of autologous vein as interposition vein graft was first described in the last century [6], but the debate on flap-failure rate associated with vein grafts still exists [7]. Moreover, approximately 30–40% patients did not receive suitable autologous vessels as grafts [8]. Despite use of the autologous vein, cryopreserved allogenic vascular graft is being investigated as a vascular conduit in reconstructive microsurgery, with most pre-clinical results showing similar patency rates as autologous vein grafts in short-term follow-up [9–13]. Currently, cryopreserved allografts (arteries or veins) of relatively large caliber are being commonly used in cardiovascular surgery and liver transplantation [14, 15]. Positive outcomes were reported in the treatment of mycotic aneurysms, infected vascular prostheses, peripheral arterial bypass, reconstitution of arteriovenous fistula, and hepatic/portal vein reconstruction [16–20]. However, clinical reports on cryopreserved vascular grafts in microsurgery are extremely rare [21].
Recent advances in cryobiology have enabled the controlled freezing of tissues with the preservation of vital cellular elements, providing cryopreserved allogenic valves and vessels for various clinical uses [22, 23]. The cardiovascular tissue bank of Taipei Veterans General Hospital is a Taiwan Food and Drug Administration certified institute, established to follow Good Tissue Practices (GTP) and Good Manufacturing Practices regulations. The cryopreserved homografts were prepared in GTP-level cardiovascular tissue bank of Taipei Veterans General Hospital, following routine processes of recovery, processing, decontamination and cryopreservation that are compliant with the American Association of Tissue Banks standards. Besides, the grafts were obtained from cadaveric donors who were brain-dead but the heart was still pumping. After minimal manipulation and antibiotic sterilization, the vascular grafts were placed in frozen medium to ensure good cell viability in the lumen of the grafts and prevent thrombosis [24]. Here, we used cryopreserved allogenic vessels in a free-flap reconstruction surgery for a complex soft tissue defect of the lower limb. Postoperative flap perfusion was uneventful with satisfactory wound healing.
SURGICAL PROCEDURE AND OUTCOME
A 54-year-old male was admitted to our ward for necrotizing fasciitis of the left lower limb. The infection was controlled gradually after emergent fasciotomy, serial debridement and wound care. Free anterolateral thigh (ALT) flap was used to reconstruct the exposed lateral aspect of the left knee joint and patella (Fig 1A). Intraoperatively, the recipient vessels were explored over the left medial thigh away from the injury zone. One arterial branch from the superficial femoral artery with its vena comitans and another branch from the great saphenous vein were identified and prepared as recipient vessels. However, the short pedicle of the ALT flap was noted after delivery through the subcutaneous tunnel, and vascular conduits were needed. Two segments of the great saphenous veins (~5 cm length, ~1.5 mm diameter) were harvested for bridging the artery and vein. One segment of the cryopreserved hepatic artery (~3 cm length, ~2–3 mm diameter; Fig 1B) was applied to bridge another large-sized vena comitans (~3 mm diameter) toward the great saphenous vein branch (Fig 1C, yellow arrows indicated the anastomotic sties of the cryopreserved vascular graft). After completing six anastomoses, the blood flow was confirmed by Doppler and bleeding observed from the flap edge, with no ischemia or congestion. The flap was then inset and the wound was closed smoothly by placing a Jackson-Pratt drain. One week postoperatively, the flap condition was stable with satisfactory healing (Fig 2A, some breakdown of flap skin was due to previous skin graft harvest). The color Doppler demonstrated patency of all three grafts (Fig 2B demonstrated the blood flow of cryopreserved vascular graft). 2-month follow-up showed healing of the wound (Fig 2C).
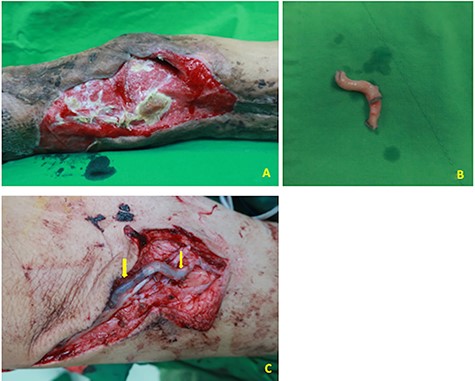
(A) A case of lower extremity necrotizing fasciitis after treatment with soft tissue defect at lateral aspect of the left knee with exposed joint and patella. (B). One segment of the cryopreserved hepatic artery (~3 cm length, ~2–3 mm diameter) at back table. (C) Cryopreserved hepatic artery was used to bridge one large-sized vena comitans (~3 mm diameter) toward the great saphenous vein branch (yellow arrows indicated the anastomotic sties of the cryopreserved vascular graft).
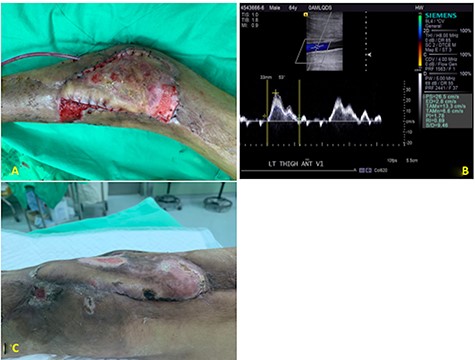
(A) The flap condition was stable with satisfactory healing at 1 week. (B) The color Doppler demonstrated the patency of cryopreserved vascular graft. (C) The wound condition with complete healing by the flap coverage at 2 months.
DISCUSSION
This case demonstrated the use of small-caliber cryopreserved vascular graft in microsurgery. According to pre-clinical reports and our animal study experience [9, 11, 25], short-term patency of allogenic grafts provides temporary circulation for flap survival. However, our case used the cryopreserved vascular graft to bridge the vein and not the artery, because of the recipient artery’s size discrepancy. The hepatic artery used was obviously larger than the recipient artery (branch of the superficial femoral artery). Anastomosing a small-sized artery to a large-sized artery causes thrombosis due to turbulence.
The intact endothelium of small-caliber blood vessel is crucial for successful reconstruction and patency improvement [23]. Although several steps affect preservation of cellular viability during handling of cryopreserved vascular grafts (e.g. preservation technique), recovery of fresh homograft is considered important. In our tissue bank, vascular grafts were harvested from brain-dead donors within 2 hours before asystole, thus maintaining more live cells in the grafts.
Although using vascular grafts in reconstructive surgery is rare, the vascular grafts could provide tension-free anastomoses when recipient vessels were short, diseased or damaged [5, 26, 27]. Hence, considering the limitations of autologous grafts, cryopreserved vascular grafts could be used as small-caliber vascular conduits in reconstructive microsurgery.
CONCLUSION
This case report described a novel use of cryopreserved vascular graft in a free-flap reconstructive microsurgery. The graft maintained flow patency post-anastomosis and the flap survived well.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
Taipei Veterans General Hospital V110B-011.



