-
PDF
- Split View
-
Views
-
Cite
Cite
Takahiro Sanada, Wakako Shirai, Shota Yamamoto, Manabu Kinoshita, Naoki Tokumitsu, A case of carotid endarterectomy assisted with a three-way junction shunting tube for the internal carotid artery stenosis involving a persistent primitive hypoglossal artery, Journal of Surgical Case Reports, Volume 2021, Issue 8, August 2021, rjab362, https://doi.org/10.1093/jscr/rjab362
Close - Share Icon Share
Abstract
Only several cases of internal carotid artery (ICA) stenosis involving the persistent primitive hypoglossal artery (PPHA) have been treated with carotid endarterectomy (CEA) because of its extreme rarity. CEA was performed for an 87-year-old female with severe stenosis of the right ICA–PPHA bifurcation requiring shunting from CCA to both PPHA and ICA. We initially attempted to insert two intraluminal balloon shunts into the CCA, as previously reported. However, we found this procedure technically impossible to achieve. An improvised three-way junction tube was inserted distally into PPHA and ICA and proximally into CCA, securing blood flow during CEA. Unfortunately, the patient suffered post-operative ischemic brain lesions due to the prolonged ischemic time during our initial unsuccessful shunt attempt. A three-way junction shunting tube could be an effective shunt technique during an anatomically complicated CEA.
INTRODUCTION
Persistent primitive hypoglossal artery (PPHA) is a rare variant of the persistent carotid-basilar anastomosis, with an estimated incidence of 0.02–0.26% [1]. An aberrant bifurcation can generate hemodynamic stress in the internal carotid artery (ICA) due to the presence of a PPHA [1, 2], and hemodynamic stress impacts the progression of atherosclerosis, which is one of the causes of ICA stenosis [3]. PPHA is rarely encountered; thus, only several cases treated with carotid endarterectomy (CEA) have been reported. Most patients were treated using intraoperative shunting from common carotid artery (CCA) to PPHA or ICA [2, 4–8]. Here, we report a case that required shunting to both PPHA and ICA from CCA via a three-way junction shunting tube.
CASE REPORT
An 87-year-old female presented left homonymous hemianopsia and hemiparesis. She had a past medical history of hypertension and dyslipidemia. Diffusion-weighted imaging (DWI) in magnetic resonance imaging (MRI) showed infarction suggestive of high signal intensity lesions at the border zones between the anterior and middle and the middle and posterior cerebral artery territories (Fig. 1A). It also showed multiple infarctions in the territory of the posterior circulation. Computed tomography angiography (CTA) revealed severe stenosis, which nearly occluded the ICA bifurcation just proximal to the right ICA–PPHA bifurcation. The ipsilateral vertebral artery was absent, and the contralateral VA was rudimentary (Fig. 1B). PPHA originated from the posterior wall of the ICA at the C1–C2 intervertebral space and passed the hypoglossal canal (Fig. 1C). The basilar artery was supplied only by PPHA, and the anterior communicating artery and posterior communicating arteries were absent. CEA was planned to prevent further cerebral infarctions.
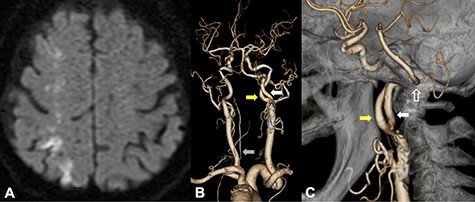
DWI on MRI showed infarction suggestive of high signal intensity lesions at the border zones between the anterior and middle and the middle and posterior cerebral artery territories (A); CTA from a posterior view revealed that PPHA (white arrow) originated from the posterior wall of the ICA (yellow arrow); the ipsilateral vertebral artery was absent, and the contralateral VA was rudimentary (B, gray arrow); CTA from a medial view revealed that PPHA entered the cranium through the hypoglossal canal (C, white line arrow).
The patient was put under general anesthesia with nasotracheal intubation. Somatosensory evoked potential (SEP) recordings were performed with left median nerve stimulation (rate: 10 Hz, intensity: 30 mA, duration: 0.2 ms). The right parietal electroencephalogram (EEG) needle-electrodes were placed at CP4 according to the International 10-20 System, and EEG were recorded using Fz electrode as reference. The head was rotated 30° to the left side, extending the right anterior neck, and a skin incision was performed (Fig. 2A). The posterior belly of the digastric muscle and the occipital artery were ligated and dissected. The styloid process was also removed. The hypoglossal nerve, the ICA, the PPHA, the CCA and the external carotid artery were exposed (Fig. 2B). After arterial cross-clamping of the arteries, arteriotomy was performed. We first attempted to insert two dual intraluminal balloon shunts in the CCA (Furui’s shunt; Inter Medical Co, Ltd, Nagoya, Japan) to individually secure arterial flows from CCA to ICA and CCA to PPHA, as previously reported [9]. We found, however, that this surgical procedure was technically challenging to achieve. Therefore, we improvised to connect two shunt tubes to form a three-way junction tube. The shunt tube was inserted distally into PPHA and ICA and proximally into CCA, securing arterial flow during endarterectomy (ischemic time of PPHA: 32 min, ICA: 52 min) (Fig. 2C and D). Although the N20-P25 amplitude of the SEP became nearly flat during cross-clamping of the ICA, the N20 amplitude moderately recovered after shunt placement and sustained until the end of the CEA (Fig. 3). Although new ischemic brain lesions in the right ICA territory appeared on DWI 1 day after surgery (Fig. 4A), CTA demonstrated favorable patency of all arteries (Fig. 4B).
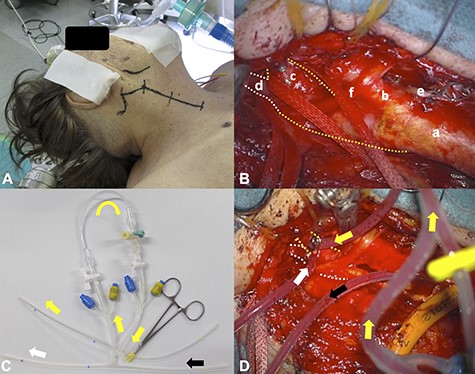
The surgical set-up is shown; the skin incision was performed along the anterior rim of the sternocleidomastoid muscle, slightly changing its direction at the right mandibular angle to the mastoid process (A); the right CCA (a), ECA(b), ICA (c, yellow dotted line), PPHA (d, white dotted line), superior thyroid artery (e) and hypoglossal nerve (f) were exposed (B); the three-way junction tube shunted the blood flow from the right CCA (black arrow) to ICA (yellow arrow) and PPHA (white arrow); the ICA (yellow dotted line) and PPHA (white dotted line) are shown (C and D).
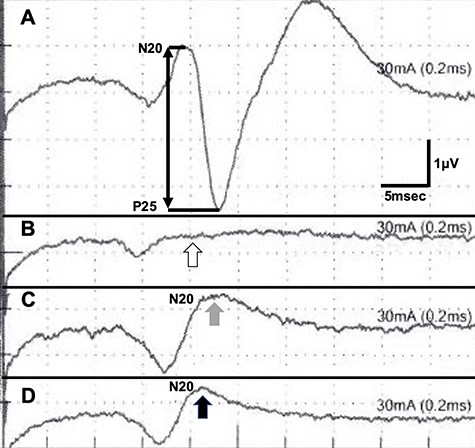
SEP recordings during CEA (A, before cross-clamping of the ICA; B, during cross-clamping; C, after shunt placement and D, at the end of CEA); the N20-P25 amplitude diminished during cross-clamping (B, white arrow); it recovered after shunt placement (C, gray arrow and D, black arrow).
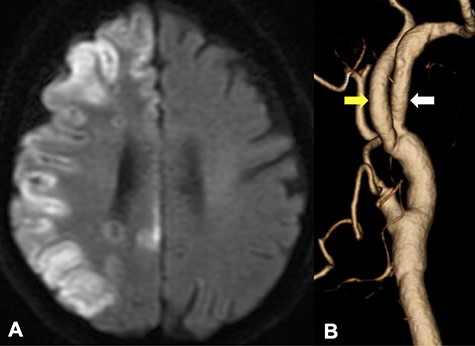
DWI in MRI demonstrated the new ischemic brain lesions on DWI 1 day after CEA in the right cerebral hemisphere cortex of the right ICA region (A); CTA demonstrated favorable patency of all arteries with an improvement of the internal carotid stenosis (B, yellow arrow: ICA, white arrow: PPHA).
DISCUSSION
Intraoperative shunting can keep ischemia time to a minimum during a CEA procedure. Most cases reported intraoperative shunting from CCA to PPHA without shunting ICA when PPHA was present [2, 5, 7, 8]. When PPHA is present, VA or posterior communicating arteries may be hypoplastic or absent due to the anatomical features of PPHA [10]. The circle of Willis was not intact, and the posterior circulation depended on PPHA for this case. Moreover, while a decrease of N20-P25 amplitude >50% is an absolute indication for intraoperative shunting during CEA, N20-P25 amplitude became nearly flat during cross-clamping of ICA in the present case [11, 12]. These conditions suggested that this patient suffered low ischemic tolerance, and shunt placement for PPHA was necessary during the CEA procedure. Therefore, shunts had to be to both PPHA and ICA from CCA for this specific patient.
Although a previous report suggested that dual intraluminal balloon shunts were an effective surgical procedure for CEA with primitive proatlantal intersegmental artery [9], we found this technique to be unrealistic. As the balloons inflate concentrically within CCA, one of the tubes pushed out the other, and securing the blood flow of both CCA tubes was impossible. The post-operative cerebral infarction was probably caused by the prolonged ischemic time while we were trying this unfruitful procedure. When the patient has an arterial anomaly, such as in this case, a three-way junction shunting tube could be a more effective solution, which secures adequate arterial flows during endarterectomy and poses much fewer technical issues than dual shunt placement into CCA.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
The study was funded by the Japan Society for the Promotion of Science (Grant-in-Aid for Early-Career Scientists No.19K18374).



