-
PDF
- Split View
-
Views
-
Cite
Cite
Emanuel Mejías-Lafontaine, Sandra Galarza, Ivan Gonzalez-Cancel, Digital clubbing as first sign of giant solitary fibrous tumor. A case report, Journal of Surgical Case Reports, Volume 2021, Issue 8, August 2021, rjab337, https://doi.org/10.1093/jscr/rjab337
Close - Share Icon Share
Abstract
Solitary fibrous tumors are rare pleural tumors. Most of the time they are benign tumors and identified incidentally once they cause symptoms secondary to their mass effect. Here we present an interesting case of a 54-year-old female found with a giant solitary fibrous tumor with signs of hypertrophic pulmonary osteoarthropathy with associated digital clubbing 6 month before identifying the tumor. The initial percutaneous biopsy revealed pathologic findings consistent with benign solitary fibrous tumor, but after complete mass excision diagnosis was upgraded to a malignant solitary fibrous tumor. Percutaneous biopsy results should not guide therapy in these patients; this is why complete excision continues to be the treatment of choice.
INTRODUCTION
Solitary fibrous tumors (SFTs) of the pleura are a rare entity of thoracic neoplasms and account for <2% of all soft tissue tumors [1]. These were first described in 1870, by Wagner, and then Klemperer and Rabin were the first who published the accurate pathological description of this tumor in 1931, classifying them as either ‘localized’ or ‘diffuse’ mesothelioma. These soft-tissue neoplasms arise from pluripotent fibroblastic or myofibroblastic origin, may arise anywhere throughout the body. Most of the time they are found incidentally or after workup due their mass effect.
CASE REPORT
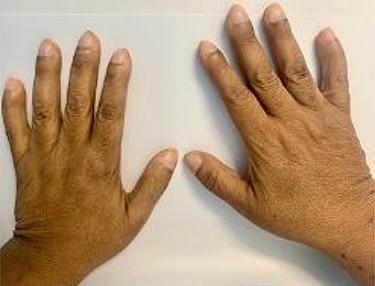
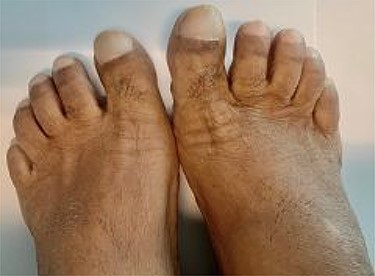
This is the case of a healthy 54-year-old female with a 3 month history of worsening dyspnea on exertion, nonproductive cough, general malaise and arthritic symptoms of her distal joints. Also, with an associated new onset, 6-month history of clubbing of her nails. She denied smoking history, weight loss, fever or chills. On examination she was found with clubbing of her nails (see Figs 1 and 2), and absent right thoracic breath sounds. Initial Chest X-ray (CXR) revealed a right side intrathoracic mass occupying the right thoracic cavity (see Fig. 3). She underwent a Chest computerized tomography (CT) scan with intravenous contrast revealing a large heterogenous mass 19 cm × 16 cm × 15 cm without gross calcifications (See Fig. 4). Then a CT-guided core needle biopsy was performed and was consistent with a benign SFT. In view of the mass size and worsening dyspnea, mass excision was performed by a single right side thoracotomy.
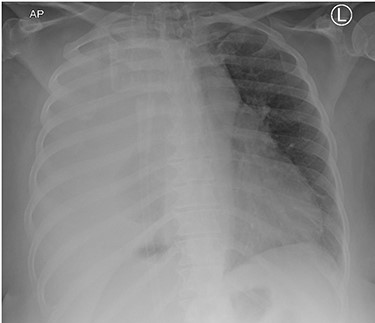
Initial CXR with compressed right lung, possible mass lesion and pleural effusion.
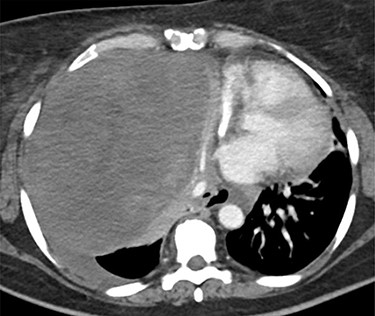
Chest CT scan. Large heterogenous mass in the right hemithorax, displacing the heart, great vessels and trachea, and esophagus to the left of midline. The origin may be hilar. No definite calcifications. Unrelated to vessels, and with associated left pleural effusion.
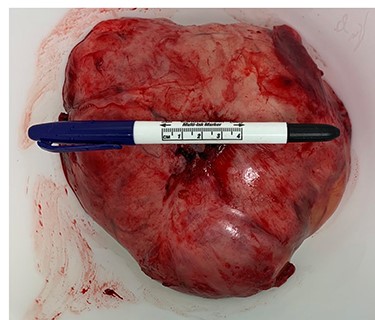
Surgery consisted of a right thoracotomy through the fifth intercostal space. The mass was found to be compressing the three lung lobes of the right lung, and no gross mediastinal invasion appreciated. Mass excised using a combination of blunt dissection and cauthery. An anterior and posterior thoracostomy tubes were left in place. Final dimensions of the mass were 19.2 cm × 17.5 cm × 10.5 cm (See Fig. 5). After mass excision right lung collapse was evident (See Fig. 6). Then after right lung ventilation, all three right lobes expanded (See Fig. 7). Postoperative CXR was also consistent with full right lung re-expansion (see Fig. 8).
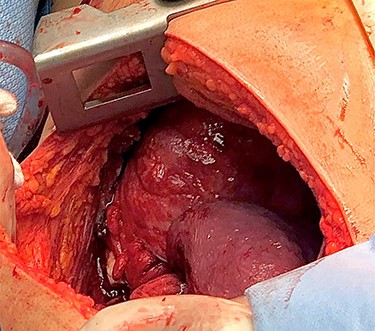
Right thoracic cavity after mass excision showing complete right lung collapse.
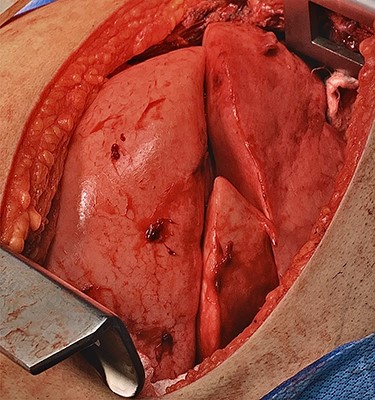
Right lung complete re-expansion of all three lobes after starting right lung ventilation.
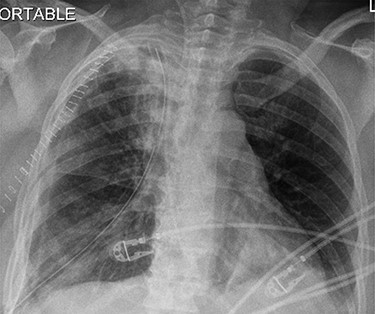
Postoperative CXR revealing complete lung expansion, no associated pneumothorax, no effusion.
Pathology report described the mass external surface as tank-pink, and upon sectioning a focal red-brown fleshy area with remaining surface of heterogeneous white-tan-pink areas of degeneration. Tumor described as a patternless architecture of spindle cells with dense collagen fibers and hypercellular areas of marked atypia, highly pleomorphic nuclei, tumor necrosis and 32 mitoses per 10 high power fields (HPF); all features were consistent with malignant SFTs. The CD34 and CD99 markers revealed diffuse cytoplasmic staining of neoplastic cells. The STAT6 revealed diffuse nuclear staining of neoplastic cells. Also, the Ki-67 was positive for nuclear staining and with a proliferation index of up to 20%.
DISCUSSION
SFTs have an estimated frequency of 2.8 cases per 100 000 patients. Only 800 cases have been reported between 1931 and 2002 [1]. Even though they have been reported in all age groups, they seem more frequent between the sixth and seventh decade of life [1, 2]. Most tumors have benign features, but 10 to 20% of cases reported on literature are malignant [3].
In 2013, the World Health Organization (WHO) classified the SFTs as a fibroblast/myofibroblast tumor under the criteria for soft and bone tissue [4, 5, 7]. According to the WHO criteria, malignant SFTs are diagnosed when one or more of the following are present: infiltrative margins, hypercellularity, pleomorphism, tumor necrosis and >4 mitoses per 10 high-power fields [5–7]. On CT scan small tumors may be seen as well-defined, homogenous masses, abutting the pleural surface [1, 2]. Several CT findings may suggest malignancy, these include: a diameter exceeding 10 cm, central necrosis, unclear borders, calcifications, uneven density and ipsilateral pleural effusion [3].
Presentation will vary according to the size of the tumor. Most commonly, tumors <10 cm in diameter will be found incidentally on CXR or CT scan in asymptomatic patients [8]. Tumors larger than 10 cm tend to present with dyspnea, chest pain, fatigue and dry cough due to mass effect, as was seen with our patient. Rarely, patients may present with paraneoplastic syndromes. The most common one being Pierre-Marie Bamberger syndrome (PMBS) in which patients present with digital clubbing (See Figs 1 and 2), hypertrophic pulmonary osteoarthropathy and arthralgias [9]. PMBS has a reported incidence of 35% in patients with pleural SFT. The exact physiological mechanism is not known but is believed to be associated with hepatocyte growth factors and hyaluronic acid [9]. Resolution of symptoms has been reported after pleural SFT excision.
Immunohistochemistry plays an important role during SFT diagnosis. The CD34 marker has been identified as the most common marker, and is present in ~95% of cases [1, 10]. The problem is that since this marker is present in the endothelium and vascular tumors, it is also positive for many other tumors such as spindle cell lipoma, perineurioma and dermatofibrosarcoma protuberans [1, 10]. Recently, the STAT 6 marker has been identified as one of the most specific markers for SFTs. STAT 6 belongs to the family of cytoplasmic transcription factors [10, 11]. It is hypothesized that NAB2, which is normally a transcriptional repressor, gains an activation domain when fused to STAT6 and the NAB2-STAT6 fusion gene then acts as a transcriptional activator, inducing expression of EGR target genes, resulting in increased proliferation [10, 11].
Surgery is the treatment of choice for all resectable SFTs. Thoracic SFTs have been reported with a high cure rate with an ~8% local recurrence [1] and a 5-year disease-free survival rate of about 80% [4]. Still, malignant SFTs have a reported 63% recurrence [1, 4].
CONCLUSION
This case is unique due to the rarity of these tumors and the associated paraneoplastic signs. It also highlights the importance that a benign SFT biopsy should not be conclusive, and complete resection should be performed to achieve a more accurate diagnosis and avoid underdiagnosis of a malignant tumor, as was the case of our patient.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
Nascimento LM, Gomes T, Fernandes A, Afonso A. Solitary fibrous tumors of the pleura: not always a benign entity.



