-
PDF
- Split View
-
Views
-
Cite
Cite
Yu-Chun Lu, Chung-Yu Wen, Shun-Tai Yang, Yueh-Hsun Lu, I-Chang Su, Adjunctive efficacy of intra-arterial cone-beam computed tomography angiography in the endovascular treatment of vertebra–venous fistula, Journal of Surgical Case Reports, Volume 2021, Issue 8, August 2021, rjab334, https://doi.org/10.1093/jscr/rjab334
Close - Share Icon Share
Abstract
Vertebro–venous fistula (VVF) refers to an abnormal arteriovenous shunt connecting the extracranial vertebral artery and the paraspinal venous structures. Coil embolization is the mainstay treatment of choice for VVF, and accurate definition of the endovascular target is mandatory. Traditionally, catheter-based angiograms are used for treatment planning, but those images lack bony information to delineate the precise relationship of the drainage veins to the spinal structure. Herein, we presented two VVF cases and demonstrated how we used intra-arterial cone-beam computed tomography angiography (IA-CBCTA) to determine the safe embolization zone for dense coil packing. We propose that IA-CBCTA is a useful adjunct in the endovascular planning of VVF by offering an image consisting of bony and vascular information.
INTRODUCTION
Vertebro–venous fistula (VVF) refers to an abnormal arteriovenous shunt connecting the extracranial vertebral artery (VA) and the venous structures [1]. Clinical presentations of VVFs, which are closely related to venous drainage pattern, include subjective bruit and neural compression by the engorged epidural veins [1].
Catheter-based angiography remains the mainstay for the planning of VVF embolization. Two-dimensional (2D) digital subtraction angiograms (DSA) acquisition offers a dynamic information of the fistulous angioarchitecture, but it is limited by vessel overlap. Three-dimensional rotational angiography (3D-RA) with volume-rendering (VR) images reveal the detailed fistulous angioarchitecture in a 3D view; however, it lacks bony information which is indispensable in defining the precise relationship of the drainage veins to the spinal structure.
Intra-arterial cone-beam computed tomography angiography (IA-CBCTA), derived from the unsubtracted data of the 3D-RA, displays cross-sectional images revealing positional relationships between bones and vessels. The usefulness of this tool has been advocated in many reports, [2–4] but its advantage in treating VVF is rarely addressed [5]. In this report, we aimed to delineate the utility of IA-CBCTA in the treatment strategy design for VVF embolization.
CASE REPORTS
Case 1
A 58-year-old female had a descent injury 5 months earlier. One month later, she started to have right C5 radicular pain and progressive weakness in elbow flexion. Cervical magnetic resonance (MR) imaging revealed an enlarged flow-void signal near the right C4–5 intervertebral foramen (Fig. 1A). In 2D-DSA of the right VA, a high-flow fistula connecting the right distal AV and the paravertebral vein was detected at the right C2–3 level (Fig. 1B). IA-CBCTA further delineated that the fistula connected the VA to a venous channel inside the foramen transversarium, which in turn drained through the intervertebral foramen and reached the intraspinal epidural space (Fig. 1C). We therefore set the venous compartment inside the foramen transversarium as our embolization target and spared the rest of the drainage veins.
We navigated the microcatheter (Fig. 1D) and placed a framing coil into this target. This first coil, as confirmed of its proper position by another IA-CBCTA (Fig. 1E), served as the frame for our following coiling procedure (Fig. 1F). As shown in the postop 2D-DSA and IA-CBCTA, VVF was completely obliterated, and the dense coil mass was exclusively located within the foramen transversarium, except for a single coil loop within the downstream vein (Fig. 1G and H). The patient’s neurological deficits recovered gradually, and the epidural flow-void signals disappeared in the postop MR (Fig. 1I).
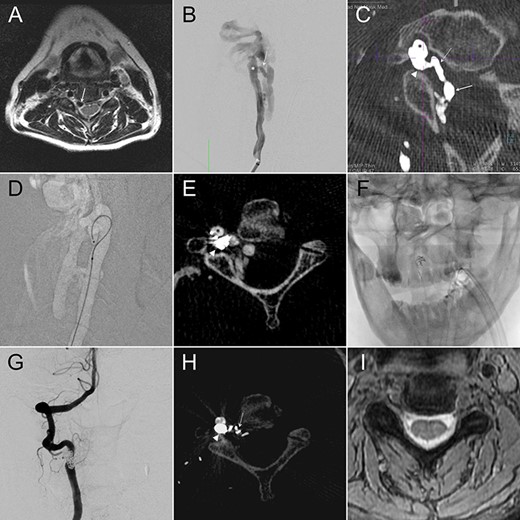
(A) Cervical MR revealed an enlarged epidural flow-void signal near the right C4–5 intervertebral foramen. (B) Anteroposterior (AP) view of the right VA angiography demonstrated a high-flow VVF at the C2–3 level. (C) An oblique axial reconstruction of IA-CBCTA revealed the detailed angioarchitectural arrangement of the VVF, as well as its anatomical relations to the foramen transversarium, intervertebral foramen and the spinal canal. The proposed embolization target for dense coil packing was limited to the venous channel outside the intervertebral foramen. (D) Roadmap fluoroscope demonstrated the position of the microcatheter tip within the target. (E) IA-CBCTA performed after placing one framing coil confirmed the proper position of the coil. (F) This first coil therefore served as the frame for the following dense coiling under fluoroscopy. (G) AP view of the control right VA angiography demonstrated complete obliteration of the VVF. (H) Postop IA-CBCTA demonstrated the dense coil mass within the foramen transversarium, and a single coil loop within the intervertebral foramen. (I) Postop MR showed disappearance of the engorged epidural venous channels. Arrowhead, drainage vein within the foramen transversarium (the embolization target for dense coiling); Asterisk, right VA; dashed arrow: drainage vein within the intervertebral foramen; Solid arrow, drainage vein within the spinal canal.
Case 2
A 60-year-old lady presented with a pulsatile tinnitus. 2D-DSA (Fig. 2A) demonstrated a high-flow direct VVF at right C1–2 level. 3DRA-VR clearly depicted the fistula point, but numerous opacified vertebral venous plexus made anatomical interpretation difficult (Fig. 2B). Coronal reconstruction images of the IA-CBCTA revealed that the fistula connected to a venous channel located outside the intervertebral foramen, which drained through the intervertebral foramen and reached the spinal canal (Fig. 2C). This image allowed us to define the venous channel outside the spinal canal as our treatment target in which we used double microcatheter technique to perform a dense coil packing in this region (Fig. 2D) and obliterated the VVF (Fig. 2E). The ideal position of the coil mass was confirmed by the postop IA-CBCTA (Fig. 2F).
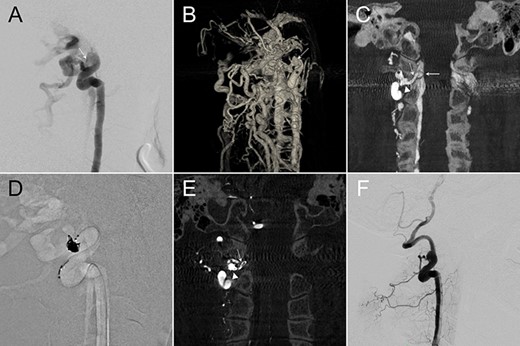
(A,B) Lateral and 3D views of the right VA angiography demonstrated a high-flow VVF at the C1–2 level. (C) An oblique coronal reconstruction of IA-CBCTA revealed the detailed angioarchitectural arrangement of the VVF, as well as its anatomical relations to the foramen transversarium, intervertebral foramen and the spinal canal. The proposed embolization target for dense coil packing was limited to the venous channel outside the intervertebral foramen. (D) Roadmap fluoroscope demonstrated the location of the dense coil mass. (E) Postop IA-CBCTA demonstrated the dense coil mass within the foramen transversarium, and a single coil loop within the intervertebral foramen. (F) Lateral view of the control right VA angiography demonstrated complete obliteration of the VVF. Arrowhead, drainage vein near the foramen transversarium (the embolization target for dense coiling); Asterisk, right VA; dashed arrow: drainage vein within the intervertebral foramen; solid arrow, drainage vein within the spinal canal.
DISCUSSION
VVF refers to a shunt connecting the extracranial VA and the vertebral venous plexus [1]. Its anatomical arrangement is characterized by the fact that the fistula is located near or within the foramen transversarium, and the associated drainage veins drain centripetally, via the intervertebral foramens, into the spinal canal [1]. The resulting arterialized venous plexus becomes dilated and results in cardinal symptoms including pulsatile tinnitus and nerve compressive symptoms [1].
Constructive endovascular embolization, including selective occlusion of the fistula without VA sacrifice, is the mainstay treatment of choice [6]. The fistula and its immediate downstream drainage vein are the embolization target; [6] however, because the drainage veins coursed intraspinally, determining a safe embolization boundaries for a dense coil packing should be individualized. If boundary is set too downstream, the coil mass will exert mass effect on the nerve roots or the spinal cord and cause unwanted compressive symptoms; in contrast, if the boundary is set too upstream it would become difficult to achieve dense coil packing without coil herniation into the parent vessel [5]. Therefore, it is mandatory to set the boundary for dense coil packing outside the intervertebral foramen, and this key step becomes much easier when IA-CBCTA technique is adopted.
IA-CBCTA can display both vascular and bony information. Without additional contrast media or radiation exposure, IA-CBCTA can be reconstructed from a routine 3DRA. As an adjunct to the traditional angiograms, this tool has been shown to increase the diagnostic power to detect intracranial microarteriovenous malformation of arteriovenous fistula [3, 4]. It is also helpful in treatment planning because of its superb ability in cross-sectional localization [2]. Similar advantage is also applicable in VVF treatment [5]. Although it is not difficult to define the fistula, the exact drainage vein location within the spine is much more difficult to define in traditional angiograms because they lack bony information. By offering bony and vascular information together, IA-CBCTA can be repeated used for embolization target selection and coil position reconfirmation.
In conclusion, IA-CBCTA, which displays exquisite vascular and bony anatomical details, is an excellent adjunct to the 2D-DSA and 3DRA-VR in defining the safe embolization boundaries for a dense coil packing of VVF.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



