-
PDF
- Split View
-
Views
-
Cite
Cite
Dema Adwan, Sami Jomaa, Red degeneration of a large leiomyoma in a non-pregnant woman in pursuit of 17 pregnancies: a case report, Journal of Surgical Case Reports, Volume 2021, Issue 7, July 2021, rjab287, https://doi.org/10.1093/jscr/rjab287
Close - Share Icon Share
Abstract
Leiomyoma is a common benign tumor in women of reproductive age. Increasing the number of pregnancy has a significant role in reducing the risk of tumor development. Red degeneration is a rare complication and often occurs during pregnancy. However, its incidence in non-pregnant women is tremendously rare. Herein, we present a case of 51-year-old non-pregnant women with a history of 17 pregnancies, diagnosed with leiomyoma that underwent red degeneration.
INTRODUCTION
Uterine leiomyoma is a benign neoplastic proliferation of smooth muscle cells of the uterus [1, 2]. As it is a hormone-dependent tumor [1], tends to arise in women of reproductive age [1, 2]. The etiology is unclear [3]; however, sex hormone, nulliparity and obesity are important risk factors [2, 4]. Contrary, increased number of pregnancy protects against tumor development [2–4]. Leiomyoma can endure hyaline, cystic, red or calcific degeneration; however, red degeneration is infrequent type and represents 3% of all leiomyomas. Compared with its incidence in pregnant women, red degeneration is extremely rare to occur in non-pregnant women [5].
In this case, we present a 51-year-old non-pregnant woman with a history of 17 pregnancies, diagnosed with intramural leiomyoma had undergone red degeneration.
CASE REPORT
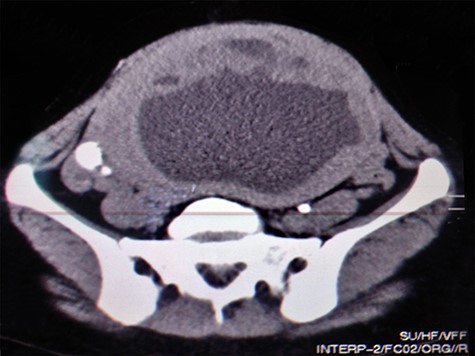
A 51-year-old woman (G 17, P 15, Ab 2) presented to the emergency department with low abdominal pain. Her gynecological history reveals pelvic pressure, irregular menstruation and menorrhagia during the past 3 months. She stated that all her pregnancies were delivered vaginally. Otherwise, neither her medical, surgical nor familial history was significant. At admission, her vital signs were in the normal range, she had mild pallor, she was afebrile and did not complain of nausea or vomiting. Her physical examination showed abdominal distention, enlarged uterine, equals to 20 weeks’ gestation. The remaining of her systemic examination was unremarkable. The patient’s laboratory tests showed microcytic anemia [RBCs: 4.42 × 106/ul, Hb: 11.2 g/dl, Hematocrit: 31.5%, MCV: 71.2 fl] and a slight increase in platelets count [403 × 103/ul] with normal white blood cell count. Her liver and kidney function were sufficient. Normal CA-125 and alpha-fetoprotein levels excluded any concerns about sarcomatous changes in leiomyoma; we were unable to perform the MRI and isoenzyme testing because they are high-priced investigations. Beta-hCG levels were normal, which exclude ectopic pregnancy. Doppler ultrasound was unavailable in our department; hence, we used normal ultrasound to rule torsion or rupture of any ovarian cysts. Both transvaginal and transabdominal ultrasound showed a large heterogeneous mass in uterine wall (Fig. 1). Pelvic CT scan reported an irregular mass measures 20 × 17 cm with central necrosis (Fig. 2).
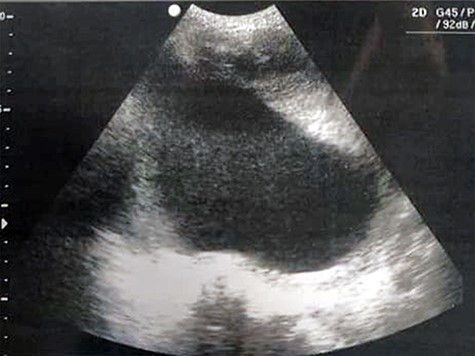
Transvaginal ultrasound showing a heterogeneous mass in the uterinewall.
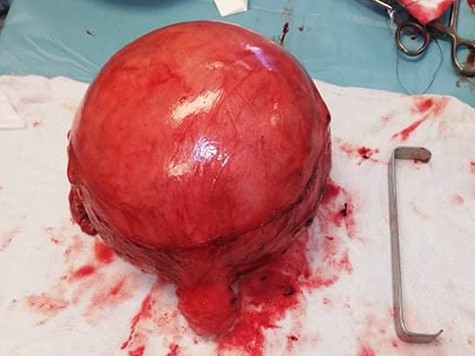
Under general anesthesia, the patient had a total hysterectomy with bilateral salpingo-oophorectomy. The gross exam showed a necrotic intramural mass measures 20 × 17 cm (Fig. 3). We implemented a biopsy to make our diagnosis, which showed atypical leiomyoma consistent with cellular leiomyoma. Congested blood vessels, areas of extensive coagulative necrosis and hemorrhages were observed (Fig. 4). She did not require a blood transfusion, and she was discharged 48 hours later after monitoring her condition.
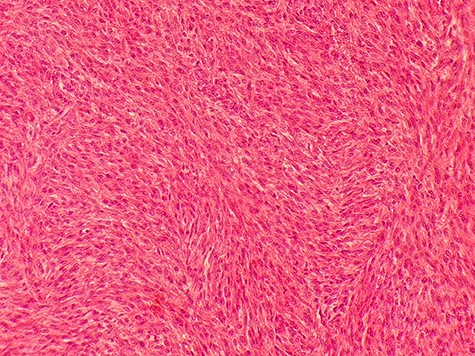
Microscopic picture shows atypical leiomyoma consistent with cellular leiomyoma with rate of mitosis <5 perHPF.
DISCUSSION
Uterine leiomyoma is the most common benign uterine tumor of women of childbearing age [1, 2]. Its incidence ranges between 50 and 60% and increases to 70–80% in women by 50 years of age [2, 6]. While estrogen has a significant role in pathophysiology [7], studies have shown a role for progesterone and its receptors [2, 6]. Risk factors of leiomyoma are sex hormones, nulliparity, early menarche, late menopause and obesity [1, 2, 4]. In contrast, risk declines as the number of pregnancies increases [2–4]; compared with a nulliparous woman, the risk of five-term pregnancies women developing leiomyoma is 25% lower [3, 4]. Our patient developed leiomyoma after 15-term pregnancies; regardless of that the risk was reduced by ~75%.
Red degeneration of leiomyoma is a rare condition records for 3% of all leiomyomas. Compared with its occurrence in pregnant women, it is tremendously rare in non-pregnant women [5]. The exact mechanisms are unclear, however, increasing tumor size resulting in inadequate blood supply, decreases oxygen delivery [5]. Besides, peripheral venous obstruction persuades hemorrhagic infarction and necrosis of the tumor [5]. Leiomyoma tolerates chronic hypoxia without revealing necrotic signs on histology, which proposes acute hypoxia as the leading cause for necrotic changes [8].
Leiomyoma is usually asymptomatic, and symptoms are related to tumor size and location in the uterus. In this case, the patient presented with excessive menstrual bleeding, which is the most common symptom [1]. Recurrent loss of pregnancies, constipation, urinary frequency and infertility are less frequent [1], and none of them reported in this case. Pain is a characteristic sign of degeneration and is usually severe, located to the tumor site and persists for 24 weeks [5]. Degeneration may cause a low-grade fever and anemia secondary to menorrhagia [5]. These symptoms are similar to those in our patient, except for the acute onset of pain, which is a rare finding. However, pain is severe, and instant management is required [7].
We performed a beta-hCG test to exclude ectopic pregnancy. Rupture or torsion of an ovarian cyst was ruled by with ultrasound; Doppler ultrasound was unavailable in our department. These differential diagnoses must be excluded in any woman with leiomyoma complains of acute abdominal pain [7]. We detected the tumor through transabdominal ultrasound; although it is useful as a primary radiological investigation [7], it has some limitations regards the tumor supply assessment for further uterine artery embolization (UAE) [1]. Furthermore, existence of hemorrhage and necrosis in leiomyoma may suggest malignancy [9]. We performed a biopsy to make our final diagnosis, which reveals atypical leiomyoma consistent with cellular leiomyoma.
Many aspects must be considered in the management of leiomyoma; size, number, location and whether the patient is willing to have children or preserve her uterus [1, 7]. Hysterectomy represents the standard surgical management for symptomatic leiomyoma [1, 7]. Although the UAE was approved as a substitute for hysterectomy [5], degeneration in leiomyomas is a contraindication for UAE; as they previously experience hemorrhagic necrosis, they will have a poor response to UAE [5]. However, leiomyoma remains the leading indication for hysterectomy [7]. We explained to our patient that although bilateral salpingectomy oophorectomy (BSO) may increase the mortality from coronary heart disease, but once BSO is integrated with estrogen therapy, the risk of death from cardiovascular disease is substantially decreased compared with women who have their ovaries preserved [10]. There is also a probability of developing osteoporosis, which can be avoided with estrogen therapy [10]. BSO also disrupts sexual function, sexual desire, arousal and orgasm; however, only sexual function can be improved with estrogen therapy [10]. Contrary, studies revealed that quality of life is improved in patients who had a hysterectomy [2, 7] and that their sexual function is not affected by the procedure [7]. Finally, BSO diminishes anxiety and depression involved in cancer risk perception [10]. She provided her informed consent and we convoyed elective bilateral salpingo-oophorectomy with total hysterectomy.
Complications of hysterectomy are related to bowel or urinary tract injury [7], and none of them occurred in thiscase.
In summary, leiomyoma is the most common benign tumor in women of reproductive age and must be considered in diagnosis even with a history of multiple term-pregnancies. Furthermore, red degeneration is a well-known complication of leiomyoma during pregnancy; it can rarely occur in non-pregnant women.
CONFLICT OF INTEREST STATEMENT
None declared.
Funding
None.



