-
PDF
- Split View
-
Views
-
Cite
Cite
Cláudia Leite, Nuno Dias, Domingos Oliveira, Rita Mesquita Pinto, Francisco Cortez Vaz, Metaplastic breast cancer with chondroid differentiation—case report and literature review, Journal of Surgical Case Reports, Volume 2021, Issue 4, April 2021, rjab113, https://doi.org/10.1093/jscr/rjab113
Close - Share Icon Share
Abstract
Metaplastic breast cancer (MBC) comprises less than 1% of all breast cancers, and it is defined by a mixture of adenocarcinoma plus mesenchymal and epithelial components. It is more common in older and black female patients. It has a larger size and faster growth, and it is frequently node-negative and triple-negative when compared with invasive ductal carcinoma. The authors report the case of a 72-year-old female patient, presenting with a breast lump, whose biopsy revealed a probable MBC with chondroid differentiation. She underwent a breast conservative surgery (BCS) and axillary sentinel lymph node dissection (SLND). The pathological report was concordant with the biopsy, and the patient was proposed to chemoradiotherapy. Despite its rarity and more severe features at diagnosis, BCS plus SLND plus radiotherapy should be offered to these patients, associated with chemotherapy. Chondroid differentiation is the rarest of all histological subtypes.
INTRODUCTION
Metaplastic breast cancer (MBC) comprises less than 1% of all breast cancers, and it is defined by a mixture of adenocarcinoma plus mesenchymal and epithelial components.
It is more common in older and black female patients. The average age of onset is in the sixth decade [1].
MBC includes a broad variety of histological patterns, such as spindle-cell carcinoma, carcinosarcoma, squamous cell carcinoma of ductal origin, adenosquamous carcinoma, carcinoma with pseudosarcomatous metaplasia, matrix-producing carcinoma, etc.
It has a larger size and faster growth, being frequently node-negative (in around 70%), triple-negative (in more than 80%) and metastatic when compared with invasive ductal carcinoma [2].
CASE REPORT
The authors report the case of a 73-year-old female patient with complaints of a left breast lump, referred to our Breast Clinic, due to a suspicious nodule of 25 mm in the upper external quadrant (UEQ) of the left breast, without suspicious axillary lymph nodes on breast ultrasound (US) (Fig. 1) and mammogram. Menarche and menopause occurred at 10 and 51 years old, respectively. Her menstrual cycles had been regular. She had had three gestations and three normal deliveries after which she breastfed. She had taken oral contraceptives but no hormone replacement therapy. She had a history of alcohol consumption and had no relevant family history. At our clinic, upon examination, she had a suspicious lump of around 30 mm in the UEQ of the left breast. The breast micro-biopsy revealed a breast tumor with abundant chondroid stroma component with necrotic areas; cells were small with an increased nucleus-to-cytoplasm ratio, hyperchromatic nuclei, positioned in a cordonal pattern (probable metaplastic carcinoma). Concerning immunohistochemistry, it was positive for AE1/AE3 and negative for BCL2, CD34 and P63. This case was presented at our Multidisciplinary Breast Tumour Board and surgery was proposed. Thus, she underwent a breast conservative surgery (BCS) (wire-guided quadrantectomy) (Fig. 2) and axillary sentinel lymph node dissection (SLND) (following de Z0011 protocol), which ran uneventfully. Intra-operative frozen section revealed negative microscopic surgical margins and three axillary lymph nodes negative for macro-metastasis. Metallic clips (of titanium) were applied in the margins of the quadrantectomy. She had an uneventful recovery and was discharged home on the third post-operative (PO) day. The definite pathological report (Fig. 3) revealed an invasive breast carcinoma, of metaplastic type, with mesenchymal differentiation (chondroid), intermediate (2) grade, triple-negative, with 31-mm, negative microscopic surgical margins and three axillary lymph nodes negative for metastasis, pT2N0 (sn). The tumoral lesion was a proliferation with infiltrative margins, presenting a peripherical component of trabecular and cordonal pattern, composed by small cells with ill-defined margins and hyperchromatic nuclei. Pleomorphism was moderate, exhibiting relatively frequent mitosis’ figures. It exhibited transition to a matrix of chondroid features, centrally positioned, abundant, with features of maturity. Peripherally, there were no tumoral veno-lymphatic emboli, images of peri-neuronal infiltration nor necrotic areas, but there was a component of in situ carcinoma. Concerning immunohistochemistry, it was positive for vimentin, CK 5/6 and 7, p63 and GATA3, but negative for smooth muscle actin, calponin, CK 20, S100 protein, hormonal (estrogen and progesterone) receptors and human epidermal growth factor receptor 2 (HER2). The proliferative index, assessed by the Ki67, was of 80% on the trabecular component. She had a thoracic, abdominal and pelvic computed tomography done, which did not show any additional disease. This case was presented again at our Multidisciplinary Tumour Board, where chemoradiotherapy was proposed. The chemotherapy protocol proposed was 4 cycles of doxorubicin 96 mg and cyclophosphamide 965 mg each 21 days, followed by 12 cycles of weekly taxane.
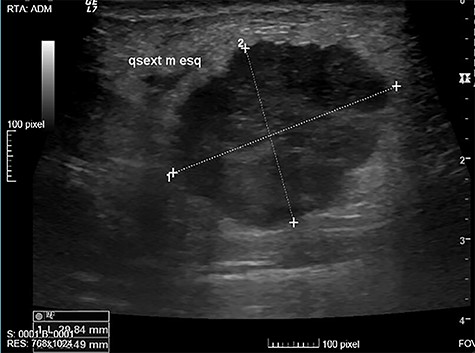
Suspicious nodule of 25 mm, in the UEQ of the left breast on breast US.
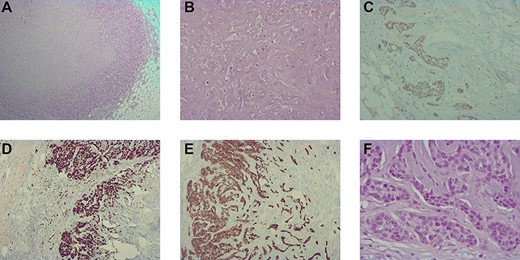
(A) histological image (hematoxylin–eosin staining, 40×) showing the trabecular and cordonal peripherical component; (B) histological image (hematoxylin–eosin staining, 200×) showing the mature chondroid central component. (C) immunohistochemistry image (100×) showing a heterogeneous and weak positivity for GATA3 in the peripherical component; (D) immunohistochemistry image (100×) showing a high positivity for Ki67; (E) immunohistochemistry image (100×) showing positivity for CK 7 in the peripherical component and (F) histological image (hematoxylin–eosin staining, 400×) showing a moderate nuclear pleomorphism with figures of mitoses in the trabecular and cordonal peripherical components.
DISCUSSION
MBC comprises around 0.4% of all breast cancers [1]. However, it accounts for a significant proportion of the global breast cancer mortality.
MBC exhibits a stem-cell-like phenotype, with innate plasticity supporting differentiation into heterologous elements. It frequently shows molecular alterations in epithelial-to-mesenchymal transition, activation of the genes of PI3K-AKT-mTOR, RTK-MAPK, nitric oxide and Wnt/β-catenin signaling pathways, altered immune response and cell cycle dysregulation.
MBC is considered to convey a worse prognosis. Five-year and 10-year overall survival is around 63 and 50%, respectively [1]. Overall and disease-free survival in MBC is similar to triple-negative ductal breast cancer [2].
MBC is poorly responsive to neoadjuvant treatment, more resistant to standard chemotherapy, and surgery remains the first choice [2].
Overall survival is improved with radiotherapy and chemotherapy regardless of the stage [3].
Immunotherapy targeting Programmed Death Ligand 1 (PD-L1), immune-suppressing T cells (Tregs) and FOXP3 may be effective, even for metastatic disease [4, 5].
Distant metastases of MBC have been described to occur in around 25% of patients, whereas local recurrences are rare (in only 7%) [6]. MBC is associated with fewer bone and liver metastasis cases but more lung metastasis cases when compared with invasive ductal carcinoma. Inactivation of HER-2/CXCR4/Akt signaling pathway contributes to the formation of lung metastases, while its activation contributes to the formation of bone and liver ones [7].
Concluding, MBC is very uncommon and has more severe features at presentation. Yet, BCS plus SLND plus radiotherapy should be offered to these patients, as it is the standard of care for early breast cancers, associated with chemotherapy. Chondroid differentiation remains the rarest of all histological subtypes.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr Teresa Dias Carvalho from Pathology, Centro Hospitalar Tondela-Viseu for her support on the availability of the histological images.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



