-
PDF
- Split View
-
Views
-
Cite
Cite
Kantaro Hara, Yumi Matsuda, Nao Furukawa, Kohshi Nagano, Metachronous multiple pulmonary nodules 9 years after esophagectomy: a case report, Journal of Surgical Case Reports, Volume 2021, Issue 4, April 2021, rjab096, https://doi.org/10.1093/jscr/rjab096
Close - Share Icon Share
Abstract
A 79-year-old man was referred to our hospital for further examination. He had undergone radical esophagectomy with right thoracotomy 9 years ago. Four cycles of chemotherapy (CDDP +5-FU) were also performed for him. Eight years after esophagectomy, two nodules were identified in the upper lobe of the right lung on chest computed tomography (CT). Owing to the possibility of new primary lung cancer, partial resection was performed. Histopathological examination revealed squamous cell carcinoma. One year and two months later, follow-up chest CT scan revealed a nodule shadow of 1.5 cm in the left apex and a nodule shadow of 0.9 cm below the S9 pleura. Hence, partial left lung resection was performed. Five months after left lung resection, a metastatic liver tumor was found on abdominal CT and left lobectomy of the liver was performed. One year after hepatectomy, the patient died due to peritoneal dissemination.
INTRODUCTION
We often encounter cases in which pulmonary nodules appear after esophagectomy. The differentiation between lung metastasis from esophageal cancer and a second cancer is required since it greatly contributes to treatment decision-making. We have reported a case in which bilateral metachronous pulmonary nodules were found after radical esophagectomy.
CASE REPORT
A 79-year-old man underwent right subthoracic esophagectomy and posterior mediastinal reconstruction for esophageal cancer (squamous cell carcinoma, pT3N4M0 Stage IVa) 9 years ago. The patient had previously undergone four cycles of CDDP+5-FU. After esophagectomy, the patient developed ileus three times; it was conservatively managed. During the third hospitalization, chest computed tomography (CT) showed a circular nodule measuring 1.4 cm in the S1 apex of the right upper lobe and a nodule measuring 0.9 cm outside S2 (Fig. 1). No findings were suggestive of esophageal cancer recurrence. 18F-Fluorodeoxyglucose-positron emission tomography revealed FDG uptake increated in the nodule in the right lung S1 with a maximum standardized uptake value of 4.3 and in the nodule in the right lung S2 with a maximum standardized uptake value of 2.7. No significant hilar or mediastinal lymph node swelling or distant metastases were detected. Bronchoscopic biopsy did not reveal any signs of malignancy. Based on the above findings, we suspected multiple lung metastases secondary to esophageal cancer, metachronous primary lung cancer or intrapulmonary metastasis secondary to primary lung cancer. We performed partial resection of the two nodule in the upper lobe of the right lung for diagnosis and treatment. Histopathological examination revealed squamous cell carcinoma (Fig. 2). Immunostaining was performed to determine whether the primary organ was the esophagus or the lung, but no diagnosis was made. On request, no additional chemotherapy was administered. Chest CT after 1 year and 2 months of right lung resection revealed a nodule measuring 1.5 cm at the apex of the left lung and a nodule measuring 0.9 cm below the S9 pleura of the left lung (Fig. 3).
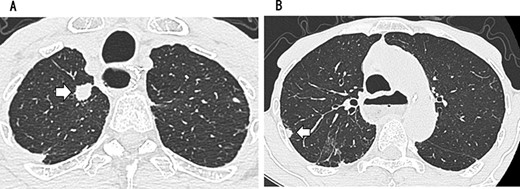
CT showing a circular nodule measuring 1.4 cm in the S1 apex of the right upper lobe (A) and a nodule measuring 0.9 cm outside S2 (B).
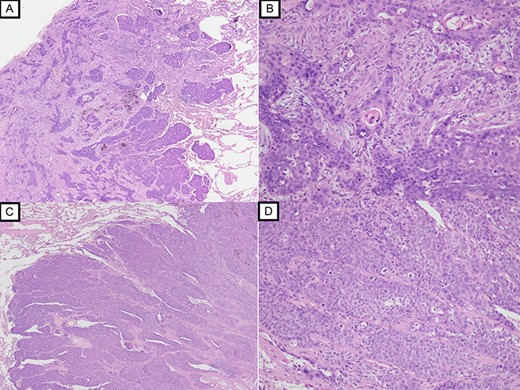
Histopathological examination with HE staining revealing squamous cell carcinoma in S1 nodule (original magnification ×20 (A), ×100 (B)); histopathological examination with HE staining revealing poorly differentiated squamous cell carcinoma in S2 nodule (original magnification ×20 (C), ×100 (D)).
Since no other findings suggestive of distant metastasis were found, partial left lung resection was performed for the two nodules. Both were diagnosed as squamous cell carcinomas (Fig. 4). Furthermore, 5 months after left lung resection, a metastatic liver tumor was found, which was also surgically resected. Finally, 10 years and 4 months after esophagectomy, 3 years after the first lung resection and 1 year after hepatectomy, the patient died due to peritoneal dissemination.
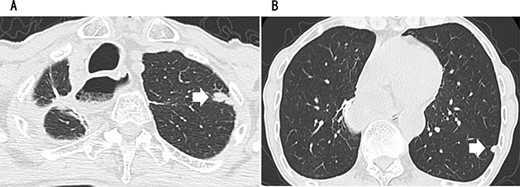
CT revealing a nodule measuring 1.5 cm at the apex of the left lung (A) and a nodule measuring 0.9 cm below the S9 pleura of the left lung (B).
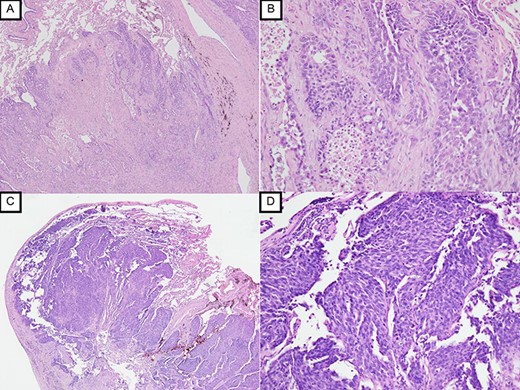
Histopathological examination with HE staining revealing squamous cell carcinoma in S1 + 2 nodule (original magnification ×20 (A), ×100 (B)); histopathological examination with HE staining revealing poorly differentiated squamous cell carcinoma in S9 nodule (original magnification ×20 (C), ×100 (D)).
DISCUSSION
Owing to recent improvements in preoperative and postoperative chemotherapy, perioperative management, esophagectomy and surgical techniques, the chances of encountering pulmonary nodules after esophageal cancer are increasing [1, 2]. It is considered that secondary cancers after esophagectomy include metachronous lung cancer and lung metastasis. The lung cancer CT screening guidelines [3] recommend a definitive diagnosis for solid nodules with a diameter of ≥10 mm or a tendency to increase, and follow-up for nodules with a diameter of 5–10 mm. In the case of peripheral lung nodules, it is difficult to make a definitive diagnosis, and surgical resection is usually performed. It is necessary to make a comprehensive judgment while considering the pathological progression of the primary lesion of the esophageal cancer, tumor markers and the presence or absence of metastases to other organs. In our case, it was difficult to make a definitive diagnosis of esophageal cancer lung metastasis or primary lung cancer despite surgical resection of both the left and right lung nodules. Although clinically it is determined as bilateral lung metastasis of esophageal cancer, it is difficult to identify the primary organ in squamous cell carcinoma.
The prognosis of recurrent esophageal cancer is extremely poor [1], and it is generally reported to be ~6 months [4]. Cases of long-term survival with local control by surgical resection have been reported, and we believe that aggressive treatment is also an option [5]. Postoperatively esophageal cancer recurrence most commonly occurs in the lungs, followed by the liver and bone [6]. Lung metastasis is the most likely target for resection.
In our case, it was concluded that the primary lesion could be controlled because it appeared from a pulmonary nodule 9 years after esophagectomy and there was no sign of recurrence until then. In addition, because the patient underwent a right thoracotomy again, we reduced the surgery to a minimum. Furthermore, because chemotherapy was rejected, we decided to consistently choose surgical resection as the local treatment. During the course, metastasis in a single organ continued in the order of the right lung, left lung and liver. Hence, surgical resection of each site was actively performed. Each surgically resected specimen revealed a diagnosis of squamous cell carcinoma. These primary lesions could not be differentiated based on pathological examination results. Nevertheless, this case in which a relatively long-term survival was achieved by surgical treatment is valuable, irrespectively of the diagnosis (multiple metastases of esophageal cancer or double cancer of metachronous lung cancer).
In conclusion, our case of metachronous multiple lung cancer 9 years after radical esophagectomy had a relatively good prognosis (10 years and 4 months after esophageal cancer, and 3 years after the first lung resection) after surgical resection alone as local therapy.
ACKNOWLEDGEMENTS
We are deeply grateful to Dr Genichirou Yoneda, Department of Diagnostic Pathology, Bell-Land General Hospital, for histopathological review of this case and providing helpful advice.
CONFLICT OF INTEREST STATEMENT
None declared



