-
PDF
- Split View
-
Views
-
Cite
Cite
Hicham Wazaren, Hanae Bouhdadi, Badre El Boussaadani, Sabrine Derqaoui, Abdelmoughit Hosni, Malick Idrissa, Chakib Benlafqih, Jaafar Rhissassi, Rochde Sayah, Mohammed Laaroussi, An unusual cause of multiple embolic strokes: cardiac myxoma, about a case, Journal of Surgical Case Reports, Volume 2021, Issue 3, March 2021, rjab063, https://doi.org/10.1093/jscr/rjab063
Close - Share Icon Share
Abstract
Cardiac myxomas are the most common primary intracardiac tumors, accounting for 50% of all cardiac neoplasms, with an estimated frequency of 0.5/million/inhabitants/year. Presenting symptoms are related to cerebral or peripheral embolism, and/or intracardiac obstruction. Thus, urgent management of myxoma is mandatory due to embolism’s risk. Herein, we report the case of an 82-year-old woman with a myxoma of the left atrium, revealed by a multiple ischemic strokes, to raise awareness of this entity.
INTRODUCTION
Cardiac myxomas are the most common primary intracardiac tumors, accounting for 50% of all cardiac neoplasms, with an estimated frequency of 0.5/million/inhabitants/year [1, 2]. Presenting symptoms are related to cerebral or peripheral embolism, and/or intracardiac obstruction [2]. Thus, urgent management of myxoma is mandatory due to embolism’s risk. Herein, we report the case of an 82-year-old woman with a myxoma of the left atrium, revealed by a multiple ischemic strokes, to raise awareness of this entity.
CASE REPORT
An 82-year-old patient presented to the department of neurology with a 6 month history of right ptosis and upper limb’s hemiparesis. In the following months, her symptoms progressed, and she developed left hemiplegia. Apart from her 5 year history of Parkinson’s diseases, she he had no major medical troubles. Upon physical examination, it was found that she was a conscious patient with stable vital signs (Blood pressure: 110/60 mmHg, heart rate: 75 bpm, temperature; 36.8°C). She had a right monoparesis, ptosis of the right eye and a 4 performance status left hemiplegia (assessed by the WHO). The heart sounds were regular without any murmurs. Peripheral pulses were present and symmetrical. Pulmonary auscultation did not find any rales and vesicular murmur was heard in both lung fields. No signs of heart failure were found.
The cerebral magnetic resonance imaging (MRI) revealed a sequelae ischemic strokes with lesions of different ages in the territory of both the left sylvic artery and branches of the basilar artery (cortical, subcortical and mesencephalic) (Fig. 1).
Transthoracic echocardiography showed a left intra-atrial mass with large implantation in the inter-atrial septum measuring 35 × 15 × 26 mm, not interfering with mitral valve movement suggestive of a myxoma (Fig. 2). The left ventricle was neither dilated nor hypertrophied with good global and segmental contractility (LVEF = 69%). Left ventricular filling pressures, right ventricle’s systolic function, were within normal ranges, with minimal mitral insufficiency. Inferior vena cava was not dilated and compliant, and the pericardium was dry. No arterial pulmonary hypertension was found. The preoperative coronary angiography was normal.
The patient was admitted to the operating room for surgical resection of the mass.
Median sternotomy was done, pericardium opened and patch harvested. Systemic heparinization 3 mg, aortabicaval cannulization, establishment of cardiopulmonary bypass along with cross-clamping of aorta was performed. The heart was arrested in diastole, right atrium opened and left atrium approached through fossa ovalis. The tumor was found to be attached to the inter-atrial septum (Fig. 3). The entire mass was excised. After thorough wash, inter-atrial septum defect was sutured with 4/0 proline. Then right atrium closure was done, cross-clamp released and heart picked up with sinus rhythm spontaneously. The patient was weaned from cardiopulmonary bypass, neutralized with protamine and decannulation, and adequate hemostasis was secured. Patient was moved to intensive care unit in stable hemodynamics.
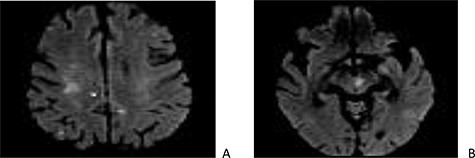
Cerebral magnetic resonance imaging (MRI): sections in diffusion at 1000 b showing punctiform cortico-subcortical (A) and mesencephalic (B) lesions related to embolic infarcts.
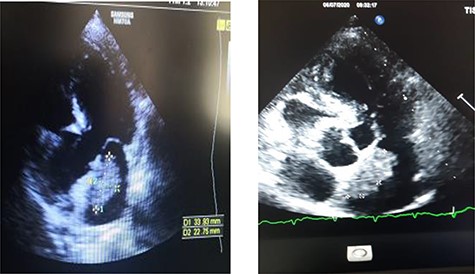
Transthoracic echographay: showing a left intra-atrial mass based on a large implantation in the inter-atrial septum.
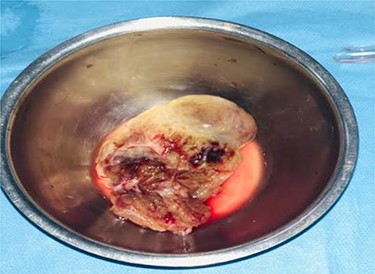
On gross pathology, the mass was friable, soft and gelatinous with multiple hemorrhagic areas. Hematoxyline and eosine stains revealed a tumor composed of stellate and globular cells arranging in cords and nests, with indistinct cell borders, abundant eosinophilic cytoplasm, round nuclei and inconspicuous nucleoli. The stroma was abundant and myxoid. Large areas of extensive hemorrhage were noticed (Fig. 4).
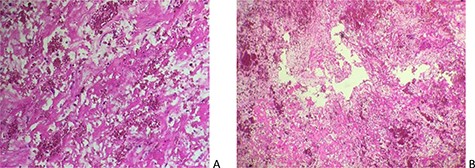
Cardiac myxoma displaying stellate cells with abundant myxoid and hemorrhagic stroma (A: Hex100, B: Hex200).
DISCUSSION
Myxoma is a benign neoplasm, accounting for the most common primary cardiac tumors. Left atrium represents the most involved site (75%), as in our patient, while 20% of the cases are located in the right atrium and less than 5% of them in a ventricle [3].
Myxoma originates from mesenchymal intracardiac embryonic remnants. It presents in two aspects: the ‘gelatinous’ form which causes enslavements of the mitral orifice, and the rounded, firm ‘fibrous’ form which is at the origin of embolic complications. It occurs sporadically; however, familial forms have been reported in 7% of cases [4].
It is usually discovered between the ages of 30 and 60 with a mild female predominance. Clinical features are dominated by the syndromic triad of left atrial myxomas (cardiac, general and embolic symptoms). In fact, embolic complications represent commonly revealing symptoms (reported in 45 to 60% of cases) and are responsible for a great morbidity and mortality. In our patient, the diagnosis was made in the course of multiple ischemic strokes [5].
Transthoracic echocardiography is a rapid, noninvasive diagnostic tool with a sensitivity approximating 95%. Transesophageal echocardiography is also useful in detecting small myxoma (sensitivity approaching 100%) or assessing the presence of a thrombus in contact with the tumor.
Surgical excision is the gold standard treatment to prevent embolic complications, which is frequent; or sudden death if the tumor is entrapped in the mitral orifice. Anticoagulant treatment is indicated before surgery; it prevents thromboembolic migration, while tumor embolism cannot be prevented.
Our patient was operated under cardiopulmonary bypass and underwent surgical excision of the mass and its implantation base.
Definitive diagnosis remains on histological analysis of resected specimen. In fact, myxomas are mesenchymal tumors resembling stromal tissue which forms vascular structures. On histology, they consist of coarsely stellate mesenchymal cells arranged in an abundant myxoid stroma.
Operative mortality is close to 1% [6]. The risk of tumor’s recurrence ranges from 4 to 7% in sporadic cases and from 10 to 21% in familial cases [7]. Electrocoagulation of the myxoma’s base and excision of a part of the healthy endocardium is indicated to prevent recurrence.
In conclusion, although left atrium’s myxomas are benign tumors, they might represent a life-threatening condition because of embolic risks. This clinical case highlights the importance of urgent surgical management of a myxoma to improve its prognosis.
AUTHORS’ CONTRIBUTIONS
All authors have read and approved the manuscript.
H.W. and M.I. are the main authors who managed the patient. H.B., S.D., B.E. and A.H. are the co-authors who analyzed the patient data and were major contributors in writing the manuscript. C.B., J.R., R.S. and ML supervised the management of the patient and revised the manuscript.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
AVAILABILITY OF DATA AND MATERIALS
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
Author notes
H. Wazaren and M. Idrissa contributed equally to this work.



