-
PDF
- Split View
-
Views
-
Cite
Cite
Michael L Williams, Charis Tan, Martin Misfeld, Tristan D Yan, Cytoreductive surgical resection of a rare pulmonary artery intimal sarcoma involving the pulmonary valve and right ventricle: a case report, Journal of Surgical Case Reports, Volume 2021, Issue 3, March 2021, rjab051, https://doi.org/10.1093/jscr/rjab051
Close - Share Icon Share
ABSTRACT
Pulmonary artery intimal sarcoma (PAIS) is an extremely rare malignant tumour. It is often misdiagnosed as chronic pulmonary thromboembolism. We describe a complex case in a 70-year-old man with PAIS extending into his right ventricle undergoing salvage cytoreductive surgical resection utilizing bivalirudin for cardiopulmonary bypass anticoagulation due to heparin-induced thrombocytopenia and thrombosis syndrome. The prognosis for PAIS is extremely poor, with a median survival of 1.5 months without surgical resection. Cytoreductive surgical debulking can improve the median survival time to 17 months. The main aim of palliative surgical resection is to improve ventilation–perfusion mismatch and prevent haemodynamic collapse.
INTRODUCTION
Pulmonary artery intimal sarcoma (PAIS) is an extremely rare malignant tumour with poor prognosis. It was first described in the literature by Mandelstamm in 1923 [1]. Since then, there have been ~300 cases reported either as case reports or small case series [2]. Due to the rarity of this disease, there is unclear evidence in the literature regarding appropriate management strategies.
CASE REPORT
We present the case of a 70-year old man who was referred to our centre for surgical resection of a PAIS. The patient initially presented with one-week history of dyspnoea, pleuritic chest pain and palpitations. The patient’s past medical history was unremarkable and was not taking regular medications prior to presentation.
The patient was initially diagnosed with a large pulmonary embolus (PE) on imaging (Fig. 1A) was thrombolyzed and commenced on intravenous heparin infusion. Subsequently, endovascular clot retrieval was performed after failing thrombolysis, where the histopathology revealed PAIS. Transthoracic echocardiogram (TTE) showed severe pulmonary hypertension with a large mass in the pulmonary artery (PA) and right ventricle (RV) with partial occlusion of the RV outflow tract (RVOT). Positron emission tomography (PET) showed glucose avidity in the RVOT and pulmonary trunk (Fig. 1B) with no nodal or extrathoracic disease. The patient was also diagnosed with Heparin-induced thrombocytopaenia and thrombosis syndrome (HITTS) and was transitioned from heparin to fondaparinux. The patient also underwent preoperative radiotherapy.
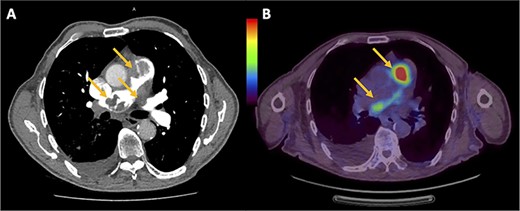
(A) Chest computed tomography pulmonary angiogram showing intraluminal filling defects in the RVOT, pulmonary trunk and left PA. (B) PET showing the uptake of fluorodeoxyglucose within the RVOT and extending into both left and right PAs.
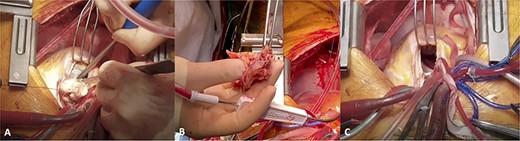
Intraoperative photos showing (A) bulky tumour being in the RVOT and main PA, (B) main part of tumour resected from PA and (C) complete cytoreduction.
Upon transferred to our centre for further work-up, a repeat TTE reported similar right heart findings as above; however, moderate-to-severe mitral regurgitation secondary to posterior mitral valve (MV) leaflet prolapse was also observed. Multidisciplinary meetings and discussions between the surgical, medical oncology and cardiac anaesthetic teams were undertaken prior to the patient undergoing surgical resection.
The operation was performed through a median sternotomy with cardiopulmonary bypass (CPB) via peripheral dual-stage venous and central aortic cannulation utilizing bivalirudin instead of heparin due to the diagnosis of HITTS. Intraoperatively, the MV was first inspected and a flail P1 leaflet along with prolapsed P2/3 leaflets was visualized. The MV was replaced with a size 31-mm St. Jude Epic bioprosthetic valve via an incision along Sondergaards’ groove. After mobilization of the pulmonary trunk from the aorta, a pulmonary arteriotomy was performed. Dense tumour load was noted in the main pulmonary trunk and both left and right main PAs (Fig. 2A) into the bronchial PAs with proximal extension into the RV. Pulmonary endarterectomy was successfully performed by developing a plane between the intima and media of the pulmonary trunk allowing resection of the tumour mass (Fig. 2B). Left and right PA endarterectomy was undertaken down to the right lower lobe arterial branches to achieve complete macroscopic cytoreduction (Fig. 2C). Resection of the tumour in the posterior aspect of the right PA involved complete resection of the adventitia, which was patched with bovine pericardium. Resection of the pulmonary valve (PV) was necessary along with resection of the tumour extending in to the RVOT and RV. PV replacement was performed with a size 29-mm St. Jude Epic bioprosthetic valve, and the RVOT was closed with bovine pericardium.
Post-operatively, the patient was commenced on milrinone and iloprost as per our pulmonary endarterectomy protocol. The post-operative course was unremarkable. Warfarin was commenced on Day 1 post-operatively, and the patient was successfully extubated on post-operative Day 2. The patient was successfully discharged on post-operative Day 7 and did not require any adjuvant therapy. On follow-up, 6-weeks post-discharge the patient was well and asymptomatic.
DISCUSSION
PAIS is a rare malignant mesenchymal tumour that arises from the embryologic bulbus cordis. PAIS usually has characteristic features of endoluminal growth, which can later lead to vessel obstruction [3]. Additionally, in even rarer cases, like the case reported, PAIS may even involve retrograde extension to the PV and even extend into the RV [4].
The disease mainly occurs in middle-aged adults with a female predominance. The estimated incidence of PAIS is thought to be between 0.001% and 0.03% [2]. However, this is likely an underestimate due to late detection at autopsy and frequent misdiagnosis for other pathologies including PE and chronic thromboembolic pulmonary hypertension [5].
The prognosis for PAIS is extremely poor, with a median survival time reported of 1.5 months without surgical resection. This increases to ~17 months with surgical intervention [2]. The role of adjuvant chemoradiotherapy largely remains undefined for the treatment of intimal sarcomas.
CONCLUSION
In conclusion, we report the case of surgical resection of an extremely rare PAIS with concomitant MV replacement, requiring alternate CPB anticoagulation strategy with bivalirudin due to HITTS. This case highlights the need for suspicion of more sinister alternate diagnoses in clinical scenarios where there is significant thrombosis found within the PA.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
REFERENCES
- anticoagulation
- bivalirudin
- cardiopulmonary bypass
- hemodynamics
- thrombosis
- thromboembolic pulmonary hypertension
- thrombocytopenia, heparin-induced
- pulmonary artery
- right ventricle
- cancer
- debulking
- pulmonary valve
- surgical procedures, operative
- palliative care
- ventilation-perfusion mismatching
- misdiagnosis
- excision
- median survival time
- pais trial
- sarcoma, intimal



