-
PDF
- Split View
-
Views
-
Cite
Cite
Faisal A AlMudaiheem, Sultan Alhabdan, Mohammed S Alhalafi, Saeed Alshieban, An insidious case of infectious mononucleosis presenting with acute appendicitis diagnosed postoperatively: a case report, Journal of Surgical Case Reports, Volume 2021, Issue 3, March 2021, rjab039, https://doi.org/10.1093/jscr/rjab039
Close - Share Icon Share
Abstract
Infectious mononucleosis (IM) is a syndrome caused by the Epstein-Barr virus. IM typically presents with fever, pharyngitis and lymphadenopathy. Rarely, it can cause acute appendicitis. We report the case of a 19-year-old female presenting with a chief complaint of colicky, non-radiating abdominal pain for 2 days. Abdominal examination revealed rebound tenderness in right iliac fossa tenderness and splenomegaly. The diagnosis of acute appendicitis was confirmed by computed tomography. She underwent laparoscopic appendectomy and mesenteric lymph node biopsy. She was later diagnosed with IM based on laboratory findings and histopathology results. She received a course of intravenous acyclovir and was discharged. This shows that IM may present with acute appendicitis as the initial presentation and may not be accompanied by any other significant symptoms of IM.
INTRODUCTION
Infectious mononucleosis (IM) is a common syndrome that typically manifests with fever, pharyngitis, lymphadenopathy and splenomegaly, and is caused by the Epstein-Barr virus (EBV) [1, 2]. The diagnosis of IM is established through history, physical examination and laboratory findings [1]. Abdominal pain is an uncommon presenting symptom for IM, but when present can be attributed to mesenteric lymphadenitis, splenic and liver involvement, and rarely, acute appendicitis [3, 4]. Acute appendicitis is the most common surgical emergency [5]. Appendicitis classically presents with right lower quadrant (RLQ) abdominal pain, vomiting, anorexia and localized tenderness at McBurney’s point [5].
We report the case of a young female who presented to the hospital with a clinical picture consistent with acute appendicitis. This diagnosis was confirmed by computed tomography (CT) scan and was found later to be due to underlying IM.
CASE PRESENTATION
A 19-year-old female, not known to have any previous medical or surgical history, presented to the emergency department with a chief complaint of colicky and non-radiating abdominal pain for 2 days. The pain started as generalized abdominal pain then migrated to the RLQ. Her pain was associated with non-bilious vomiting, nausea and anorexia.
On examination, the patient was afebrile, pale and dehydrated. Her vital signs were significant for tachycardia, which fluctuated between 102 and 115 bpm. The abdomen was soft, with positive dunphy’s sign, rebound tenderness at the right iliac fossa and splenomegaly. Laboratory investigations were within normal limits including a normal white blood cell count, but with left shift. Liver function tests (LFTs) were elevated and showed a cholestatic pattern (Table 1). A viral hepatitis profile was negative for active infection.
| Variable . | Reference range . | Upon admission . | Postoperative day 2 . | Postoperative day 4 . | Postoperative day 8 . | Upon discharge . |
|---|---|---|---|---|---|---|
| WBCs | 4.00–11 × 109/l | 6.44 | 8.23 | 9.84 | 6.65 | 6.17 |
| Lymphocyte | % | 45 | 50 | 50 | 44 | 61 |
| Atypical lymphocyte | % | 16 | 20 | 10 | 3 | 3 |
| EBV-IgG | U/ml | – | 60.9 | 64.2 | – | – |
| EBV-IgM | U/ml | – | >160.00 | >160.00 | – | – |
| Hgb | 120–160 g/l | 117 | 98 | 103 | 102 | 106 |
| AST | 5–34 U/l | 326 | 219 | 185 | 175 | 166 |
| ALT | 5–55 U/l | 427 | 345 | 277 | 191 | 193 |
| Alk phos | 40–150 U/l | 391 | 470 | 673 | 798 | 554 |
| GGT | 9–36 U/l | 418 | 467 | 605 | 491 | 495 |
| Bili T | < 20.6 μmol/l | 76.6 | 116.7 | 143.1 | 147 | 121.9 |
| Bili (D) | < 8.7 μmol/l | 62.4 | 87.8 | 115.6 | 112 | 91.5 |
| Variable . | Reference range . | Upon admission . | Postoperative day 2 . | Postoperative day 4 . | Postoperative day 8 . | Upon discharge . |
|---|---|---|---|---|---|---|
| WBCs | 4.00–11 × 109/l | 6.44 | 8.23 | 9.84 | 6.65 | 6.17 |
| Lymphocyte | % | 45 | 50 | 50 | 44 | 61 |
| Atypical lymphocyte | % | 16 | 20 | 10 | 3 | 3 |
| EBV-IgG | U/ml | – | 60.9 | 64.2 | – | – |
| EBV-IgM | U/ml | – | >160.00 | >160.00 | – | – |
| Hgb | 120–160 g/l | 117 | 98 | 103 | 102 | 106 |
| AST | 5–34 U/l | 326 | 219 | 185 | 175 | 166 |
| ALT | 5–55 U/l | 427 | 345 | 277 | 191 | 193 |
| Alk phos | 40–150 U/l | 391 | 470 | 673 | 798 | 554 |
| GGT | 9–36 U/l | 418 | 467 | 605 | 491 | 495 |
| Bili T | < 20.6 μmol/l | 76.6 | 116.7 | 143.1 | 147 | 121.9 |
| Bili (D) | < 8.7 μmol/l | 62.4 | 87.8 | 115.6 | 112 | 91.5 |
Abbreviations: WBCs = white blood cells; EBV-IgG = Epstein-Barr virus-immunoglobulin G; EBV-IgM = Epstein-Barr virus-immunoglobulin M; Hgb = hemoglobin; AST = aspartate transaminase; ALT = alanine transaminase; Alk phos = alkaline phosphatase; GGT = gamma-glutamyltransferase; Bili T = total bilirubin; Bili (D) = direct bilirubin.
| Variable . | Reference range . | Upon admission . | Postoperative day 2 . | Postoperative day 4 . | Postoperative day 8 . | Upon discharge . |
|---|---|---|---|---|---|---|
| WBCs | 4.00–11 × 109/l | 6.44 | 8.23 | 9.84 | 6.65 | 6.17 |
| Lymphocyte | % | 45 | 50 | 50 | 44 | 61 |
| Atypical lymphocyte | % | 16 | 20 | 10 | 3 | 3 |
| EBV-IgG | U/ml | – | 60.9 | 64.2 | – | – |
| EBV-IgM | U/ml | – | >160.00 | >160.00 | – | – |
| Hgb | 120–160 g/l | 117 | 98 | 103 | 102 | 106 |
| AST | 5–34 U/l | 326 | 219 | 185 | 175 | 166 |
| ALT | 5–55 U/l | 427 | 345 | 277 | 191 | 193 |
| Alk phos | 40–150 U/l | 391 | 470 | 673 | 798 | 554 |
| GGT | 9–36 U/l | 418 | 467 | 605 | 491 | 495 |
| Bili T | < 20.6 μmol/l | 76.6 | 116.7 | 143.1 | 147 | 121.9 |
| Bili (D) | < 8.7 μmol/l | 62.4 | 87.8 | 115.6 | 112 | 91.5 |
| Variable . | Reference range . | Upon admission . | Postoperative day 2 . | Postoperative day 4 . | Postoperative day 8 . | Upon discharge . |
|---|---|---|---|---|---|---|
| WBCs | 4.00–11 × 109/l | 6.44 | 8.23 | 9.84 | 6.65 | 6.17 |
| Lymphocyte | % | 45 | 50 | 50 | 44 | 61 |
| Atypical lymphocyte | % | 16 | 20 | 10 | 3 | 3 |
| EBV-IgG | U/ml | – | 60.9 | 64.2 | – | – |
| EBV-IgM | U/ml | – | >160.00 | >160.00 | – | – |
| Hgb | 120–160 g/l | 117 | 98 | 103 | 102 | 106 |
| AST | 5–34 U/l | 326 | 219 | 185 | 175 | 166 |
| ALT | 5–55 U/l | 427 | 345 | 277 | 191 | 193 |
| Alk phos | 40–150 U/l | 391 | 470 | 673 | 798 | 554 |
| GGT | 9–36 U/l | 418 | 467 | 605 | 491 | 495 |
| Bili T | < 20.6 μmol/l | 76.6 | 116.7 | 143.1 | 147 | 121.9 |
| Bili (D) | < 8.7 μmol/l | 62.4 | 87.8 | 115.6 | 112 | 91.5 |
Abbreviations: WBCs = white blood cells; EBV-IgG = Epstein-Barr virus-immunoglobulin G; EBV-IgM = Epstein-Barr virus-immunoglobulin M; Hgb = hemoglobin; AST = aspartate transaminase; ALT = alanine transaminase; Alk phos = alkaline phosphatase; GGT = gamma-glutamyltransferase; Bili T = total bilirubin; Bili (D) = direct bilirubin.
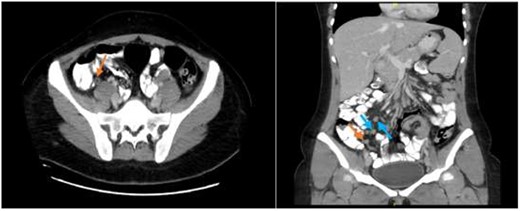
Abdominal CT scan showing an enhanced appendix, not filing with contrast and no surrounding fat stranding or collections (axial view) with associated multiple enlarged inguinal and retroperitoneal lymph nodes (coronal view).
A CT abdomen and pelvis scan was done with oral and intravenous (IV) contrast and displayed features of early uncomplicated appendicitis and multiple enlarged retroperitoneal and inguinal lymph nodes (Fig. 1).
The patient was resuscitated and started empirically on intravenous ceftriaxone, 1000 mg, once daily. Hepatotoxic medications such as IV acetaminophen, were discontinued. On the second day of admission, the patient was taken to diagnostic laparoscopy. Intraoperatively, there was early inflammation of the appendix with no signs of perforation, which was in concordance with CT scan findings. Appendectomy and an excisional biopsy of enlarged mesenteric lymph nodes at the terminal ileum were done and sent for histopathology. The procedure was otherwise uneventful.
On postoperative day 3, the patient developed multiple spikes of fever reaching up to 38.6°C. The fever did not respond to the antibiotics. Moreover, the patient developed jaundice and a concomitant rise in LFTs. Magnetic resonance cholangiopancreatography revealed active acute hepatitis with no biliary obstruction.
EBV serology along with other viral markers were requested which showed high EBV antibody titers and established the diagnosis of IM (Table 1). Histopathological results of the appendix and lymph nodes showed EBV associated changes with no evidence of acute inflammation or malignancy (Fig. 2).
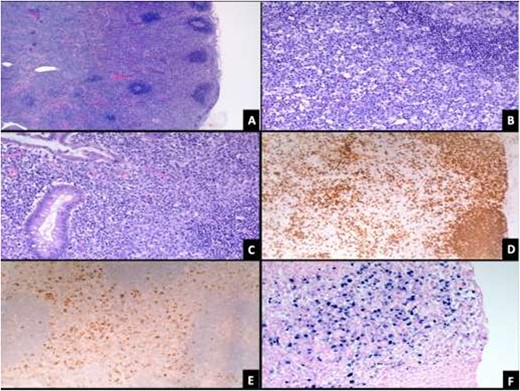
(A) Lymph node showing paracortical expansion and scattered reactive follicles (H&E stain, 50X). (B) There are numerous large lymphoid cells (mostly B-cells) with immunoblastic morphology and increased plasma cells (H&E stain, 200X). (C) Appendix shows increased immunoblasts within the mucosa (H&E stain, 200X). Immunoblasts are positive for CD20 (D), CD30 (E) and EBV-EBER (F) stains.
The patient was subsequently started on IV acyclovir, 700 mg, twice daily for 3 days. On postoperative day 9, the patient was discharged after clinical improvement on a 2-week course of oral acyclovir 200 mg, once daily. On follow up at 3 weeks, the patient demonstrated full recovery.
DISCUSSION
In this report, a case of acute appendicitis was found to be associated with underlying infectious mononucleosis. A limited number of cases in the literature documented a presentation of acute appendicitis for patients with IM. In the majority of these cases, abdominal pain was the main presenting symptom, though most patients had experienced other typical symptoms of IM before or at the time of the presentation.
In one report, a 28-year-old male presented with severe abdominal pain, vomiting and abdominal distension, with rebound tenderness of the RLQ on examination. His symptoms were preceded by an upper respiratory tract infection (URTI) 1 week prior. At appendectomy, there were multiple purulent collections and a perforated appendix [6].
In another case report, a 17-year-old male presented similarly with RLQ pain, anorexia, vomiting and signs and symptoms of URTI. Lymphadenopathy was apparent on examination. The diagnosis of IM was made before undergoing appendectomy; the additional symptoms of IM heightened clinical suspicion for EBV infection. Eventually, the patient was taken to appendectomy due to the worsening inflammation and clinical picture [4].
In our case, the patient presented with RLQ pain, anorexia, vomiting and elevated liver transaminases without any signs or symptoms of a URTI.
Appendicitis is considered a true, albeit uncommon complication of IM. This can cause a diagnostic dilemma, as the symptoms of appendicitis often predominate the clinical picture [3]. The proposed mechanism of acute appendicitis in IM is obstruction of appendiceal lumen due to swollen lymphoid tissue during the acute phase of IM, leading to a typical picture of acute appendicitis [4]. The mechanism of EBV induced cholestatic hepatitis is not fully understood; however, it is thought to be due to an immune process that involves either inflammation of the bile ducts or hepatic cell damage due to activated free radicals [7].
CONCLUSION
This report shows that IM can cause acute appendicitis and may be the initial presentation. Close clinical, laboratory and radiological monitoring of such patients is essential to attain a correct diagnosis.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
REFERENCES
- abdominal pain
- appendicitis
- computed tomography
- herpesvirus 4, human
- biopsy
- acyclovir
- fever
- ilium
- infectious mononucleosis
- pharyngitis
- splenomegaly
- diagnosis
- abdominal examination
- appendectomy, laparoscopic
- rebound tenderness
- lymph node of mesentery
- laboratory test finding
- histopathology tests
- chief complaint
- lymphadenopathy



