-
PDF
- Split View
-
Views
-
Cite
Cite
Jonathan Evans, Louis Sisk, Kee Chi, Shaun Brown, Henry To, Concurrent granulomatous mastitis and invasive ductal cancer in contralateral breasts—a case report and review, Journal of Surgical Case Reports, Volume 2021, Issue 12, December 2021, rjab519, https://doi.org/10.1093/jscr/rjab519
Close - Share Icon Share
Abstract
Idiopathic granulomatous mastitis (GM) is an uncommon chronic benign disease of the breast that is challenging to clinically distinguish from malignancy. However, it is unusual to diagnose GM concurrently with carcinoma in the same breast and rarer still to encounter GM and malignancy in contralateral breasts. We describe the case of a 39-year-old female who presented with right multifocal breast cancer and left granulomatous mastitis, discuss complexities in her management plan and review the literature on this unusual concurrent condition.
INTRODUCTION
Granulomatous mastitis (GM) is an uncommon, benign disease of the breast that is difficult to distinguish from malignancy. It typically presents with a unilateral breast mass that may be painful, with skin changes including nipple retraction, erythema and swelling. GM is frequently misdiagnosed as chronic infection or carcinoma, resulting in inappropriate management. Radiological imaging confers minimal benefit due to an irregular appearance, and diagnosis relies primarily on histopathology. Malignancy in the contralateral breast is unusual and may be an incidental finding or represent a breast field change.
The etiology of GM is poorly understood, however postulated causes include trauma, metabolic processes, granulomatous infection and autoimmunity. Genetic and hormonal changes are likely to be significant, as GM is commoner in certain ethnicities and breastfeeding women of childbearing age, usually within 5 years of pregnancy [1], and is rare in nulliparous women. There is little overlap in risk factors with malignancy, although genetic factors have been postulated.
The earliest reported link to malignancy was five sisters with chronic mastitis, three of whom developed breast cancer [2]. More recently, case reports have described concurrence of GM and malignancy in the same breast [2–7], whereas Kaviani [8] was the first to report synchronous idiopathic GM and malignancy in contralateral breasts. We present a further case report of concurrent malignancy and GM in contralateral breasts.
CASE REPORT
A 39-year-old south Asian female (AB) presented with a 5-cm left breast mass at the 10 O’clock position, increasing in size and tenderness but systemically well with no axillary lymphadenopathy. Examination of the right breast was unremarkable. Previous history included two children and a left breast abscess 1 year prior, which was aspirated under ultrasound. There was no family history of malignancy or other risk factors.
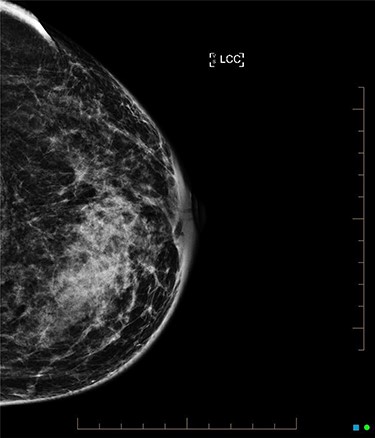
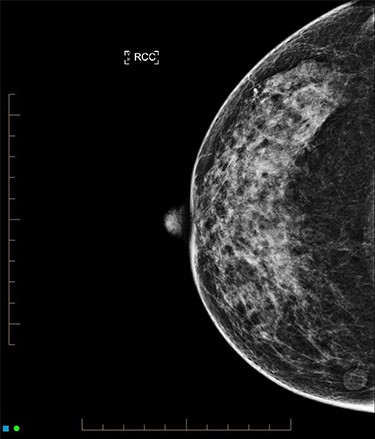
An ultrasound of the left breast demonstrated a 60-mm irregularity with no underlying collection at the 10 O’clock position, 2 cm from the nipple (Fig. 1). AB was treated with 5 days of oral flucloxacillin for presumed mastitis, and underwent a bilateral mammogram and ultrasound due to the suspicious irregularity of the left breast lesion. This showed an area of asymmetric density in the medial left breast with hyperemia (Fig. 2). The right breast showed two clusters of pleomorphic microcalcifications in the upper outer quadrant, further characterized as irregular lesions measuring 16 × 11 × 11 mm and 9 × 10 × 7 mm (Fig. 3).
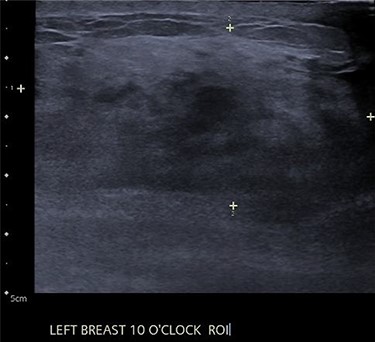
During investigations, the left breast infection worsened, resulting in a collection. Two ultrasound-guided aspirations failed, necessitating subsequent surgical drainage. Histology and of the excisional biopsy demonstrated GM with no evidence of malignancy, and no mycobacterium or Corynebacterium. With dressings the left breast fully healed.
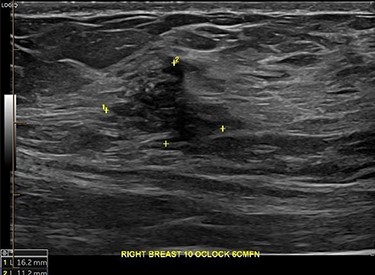
Core biopsy of the right breast demonstrated a high-grade ductal carcinoma in situ (DCIS) (Fig. 4), whilst staging computed tomography and bone scan showed no evidence of distal disease. Magnetic resonance imaging (MRI) identified a resectable 60-mm area in the right outer quadrant. AB underwent an oncoplastic right wide local excision with sentinel lymph node biopsy (SLNB), subsequently requiring an axillary dissection due to macrometastatic axillary disease. Histopathology showed multifocal high-grade invasive ductal carcinoma with immunotyping of Grade 3, PR ++, HER-2 negative, resulting in an adjuvant chemotherapy regime of cyclophosphamide and doxorubicin. The left breast was monitored without change during this time. Fully fractionated whole breast radiotherapy with boost to the tumor bed and regional lymph nodes is scheduled, alongside risk reducing endocrine therapy.
DISCUSSION
GM is an uncommon, chronic and benign condition that can mimic malignancy. Multiple etiological hypotheses include hormonal changes, autoimmunity, infection with corynebacterial or mycobacterium, smoking and as a response to trauma.
Diagnosis is often challenging. Ultrasound, mammographic and MRI findings are generally non-specific and only serve to confirm the mass, parenchymal irregularity or multifocality. As the gold standard investigation remains histology, all areas should be biopsied. For GM, chronic granulomatous inflammation is characterized by giant cells, leucocytes, epithelioid cells and macrophages [9]. Malignancy can often be delineated from this if within the analyzed specimen.
The distinction between the two disease processes remains essential as treatment differs significantly. GM treatment is primarily treated medically with antibiotics or anti-inflammatories, yet malignancy often requires multimodal adjuvant treatments (surgery, hormonal therapy, chemotherapy and radiotherapy). To distinguish between the two, it has been suggested that inflammatory markers (such as IL-33) or circulating tumor factors (miR-155, let-7c and phosphatase and tensin homolog (PTEN)) may act as future diagnostic biomarkers [10, 11].
Our case emphasizes the importance of a comprehensive evaluation of patients who present with GM. Examination and radiological investigation of both breasts should be undertaken whenever patients present with unilateral disease. Although Kaviani [8] described two distinct breast lesions presenting simultaneously, AB had a distracting presentation in one breast with occult findings of malignancy. Without diligent investigation of both breasts a significant malignancy would have gone undetected.
Management must also be carefully co-ordinated. It is important to continue aggressive management for the infection whist commencing planning and treatment for the breast cancer. Investigations should be timed and patients informed of multiple hospital visits. Multi-disciplinary discussions should also involve infectious disease specialists if bacteria are identified. Another alternative is to consider neoadjuvant chemotherapy which will provide breast cancer systemic treatment and suppress the immune system while the infection heals. Aesthetic outcomes should be considered early. Risk factors should be considered for risk reduction.
Prior cases have stimulated a discussion about the possible correlation between GM and breast cancer, but there is no causative link proven. It can be assumed that chronic local inflammation may promote malignancy, but data has indicated that despite a higher incidence of breast cancer in women with mastitis, there is no causative relationship [12]. Further research into ipsilateral GM and malignancy may demonstrate interesting results about local causative factors. In contralateral breasts, a common risk factor may emerge, providing justification to consider a systemic rather than local process.
In conclusion, GM and malignancy are rarely observed concurrently, and if present, a tailored management plan which considers aggressive infection and breast cancer treatment is required. Multi-disciplinary support is essential. A unifying risk factor is unlikely to be identified, and as it is often overlooked, careful contralateral screening is recommended for all patients with GM due to the significant management implications.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None declared.



