-
PDF
- Split View
-
Views
-
Cite
Cite
Sunil Basukala, Sabina Rijal, Saurav Karki, Bikram Basukala, Alok Raj Gautam, Spontaneous gallbladder perforation in patient with COVID-19—a case report and review of literature, Journal of Surgical Case Reports, Volume 2021, Issue 11, November 2021, rjab496, https://doi.org/10.1093/jscr/rjab496
Close - Share Icon Share
Abstract
The Coronavirus 2019 disease (COVID-19) originated in Wuhan in China in December 2019 has evolved over the past year in terms of its pathophysiology, clinical presentation, imaging manifestations and management strategies. Though COVID-19 is predominantly a pulmonary illness, it is now established to show widespread extra pulmonary involvement. Gastrointestinal manifestations of COVID-19 are also well known. COVID-19 infection presenting with the involvement of gallbladder is extremely rare in medical literature. Gallbladder perforation should be thought of in COVID-19 patients complaining of with acute abdomen in with acute cholecystitis.
INTRODUCTION
Coronavirus 2019 disease (COVID-19) has a greater preference for infecting the lower respiratory tract, with severe forms of acute renal failure (ARF) complicated by shock and acute organ failures [1–3]. SARS-COV-2 binds efficiently to the ACE2 receptors which are expressed in many organs, including the airways, lung parenchyma, several organs in the abdomen, particularly the kidneys and GI system of the body. The gastrointestinal (GI), liver and gallbladder manifestations of COVID-19 have been described in multiple studies [4, 5]. Gallbladder perforation (GBP) is a rare, but life-threatening and has high morbidity and mortality, most often due to delay in diagnosis. We hereby report a case of GBP in patient with COVID-19 managed surgically.
CASE REPORT
A 47-year-old male, known case of diabetes mellitus under oral medication, presented to emergency department with complaints of fever, mild cough and shortness of breath. The patient tested positive for COVID-19 and was admitted in COVID ward. On his sixth day of isolation following treatment for COVID-19, he complained of pain abdomen, mainly over right upper abdomen which was insidious, dull aching in nature and radiating to the back in the inter-scapular area for past 2 days following admission. On clinical examination, patient was alert, conscious, afebrile with regular pulse of 68 bpm and blood pressure 130/90 mm Hg. On examination of abdomen, Murphy’s sign was present. His laboratory parameters showed leukocytosis with raised lipase and amylase. Liver function test revealed total bilirubin 2.30 mg/dl, conjugated bilirubin 1.5 mg/dl and alkaline phosphatase 881 U/L (Table 1). His ultrasonography of abdomen revealed feature of acute cholecystitis. Hence, a provisional diagnosis of acute cholecystitis was made and patient was managed conservatively.
Table showing change in laboratory parameters on admission, during laparotomy and in post-operative period
| S. No. . | Parameters . | Normal range . | On admission . | During laparotomy . | Post-operatively . |
|---|---|---|---|---|---|
| 1. | WBC count (109 cells/L) | 3.5–9.5 109 cells/L | 13 × 109 | 19 × 109 | 8.9 × 109 |
| 2. | Neutrophil (%) | 50–70% | 73 | 79 | 83 |
| 3. | Lymphocyte (%) | 20–40% | 16 | 17 | 18 |
| 4. | Haemoglobin g/dL) | 12–16 g/dL | 13.3 g/dL | 12.1 g/dL | 11.7 g/dL |
| 5. | Platelet count (109 cells/L) | 125–350 109 | 240 | 230 | 210 |
| 6. | Haematocrit (%) | 36–48% | 42 | 44 | 40 |
| 7. | AST (U/L) | 5–45 U/L | 123 | 127 | 149 |
| 8. | ALT (U/L) | 5–40 U/L | 97 | 111 | 102 |
| 9. | Albumin (g/L) | 3.5–5.5 mg/L | 3.9 | 3.4 | 3.6 |
| 10. | Amylase (U/L) | 0–140 U/L | 98 U\L | 68 U\L | 179 U\L |
| 11. | Lipase (U/L) | 0–60 U/L | 58 | 49 | 47 |
| 12. | Blood sodium level (mEq/L) | 135–145 mEq/L | 139 | 137 | 145 |
| 13. | Blood potassium level (mEq/L) | 3.6–5.2 mEq/L | 3.9 | 3.7 | 4.1 |
| 14. | Blood calcium level (mg/dL) | 8.5–10.5 mg/dL | 6.7 | 6.9 | 7.7 |
| 15. | Triglyceride (mg/dL) | 40–150 mg/dL | 210 | 200 | 219 |
| 16. | Blood urea nitrogen (mg/dL) | 8–20 mg/dL | 50 | 110 | 120 |
| 17. | Creatinine (mg/dL) | 0.5–1.2 mg/dL | 1.2 | 1.2 | 1.1 |
| S. No. . | Parameters . | Normal range . | On admission . | During laparotomy . | Post-operatively . |
|---|---|---|---|---|---|
| 1. | WBC count (109 cells/L) | 3.5–9.5 109 cells/L | 13 × 109 | 19 × 109 | 8.9 × 109 |
| 2. | Neutrophil (%) | 50–70% | 73 | 79 | 83 |
| 3. | Lymphocyte (%) | 20–40% | 16 | 17 | 18 |
| 4. | Haemoglobin g/dL) | 12–16 g/dL | 13.3 g/dL | 12.1 g/dL | 11.7 g/dL |
| 5. | Platelet count (109 cells/L) | 125–350 109 | 240 | 230 | 210 |
| 6. | Haematocrit (%) | 36–48% | 42 | 44 | 40 |
| 7. | AST (U/L) | 5–45 U/L | 123 | 127 | 149 |
| 8. | ALT (U/L) | 5–40 U/L | 97 | 111 | 102 |
| 9. | Albumin (g/L) | 3.5–5.5 mg/L | 3.9 | 3.4 | 3.6 |
| 10. | Amylase (U/L) | 0–140 U/L | 98 U\L | 68 U\L | 179 U\L |
| 11. | Lipase (U/L) | 0–60 U/L | 58 | 49 | 47 |
| 12. | Blood sodium level (mEq/L) | 135–145 mEq/L | 139 | 137 | 145 |
| 13. | Blood potassium level (mEq/L) | 3.6–5.2 mEq/L | 3.9 | 3.7 | 4.1 |
| 14. | Blood calcium level (mg/dL) | 8.5–10.5 mg/dL | 6.7 | 6.9 | 7.7 |
| 15. | Triglyceride (mg/dL) | 40–150 mg/dL | 210 | 200 | 219 |
| 16. | Blood urea nitrogen (mg/dL) | 8–20 mg/dL | 50 | 110 | 120 |
| 17. | Creatinine (mg/dL) | 0.5–1.2 mg/dL | 1.2 | 1.2 | 1.1 |
Table showing change in laboratory parameters on admission, during laparotomy and in post-operative period
| S. No. . | Parameters . | Normal range . | On admission . | During laparotomy . | Post-operatively . |
|---|---|---|---|---|---|
| 1. | WBC count (109 cells/L) | 3.5–9.5 109 cells/L | 13 × 109 | 19 × 109 | 8.9 × 109 |
| 2. | Neutrophil (%) | 50–70% | 73 | 79 | 83 |
| 3. | Lymphocyte (%) | 20–40% | 16 | 17 | 18 |
| 4. | Haemoglobin g/dL) | 12–16 g/dL | 13.3 g/dL | 12.1 g/dL | 11.7 g/dL |
| 5. | Platelet count (109 cells/L) | 125–350 109 | 240 | 230 | 210 |
| 6. | Haematocrit (%) | 36–48% | 42 | 44 | 40 |
| 7. | AST (U/L) | 5–45 U/L | 123 | 127 | 149 |
| 8. | ALT (U/L) | 5–40 U/L | 97 | 111 | 102 |
| 9. | Albumin (g/L) | 3.5–5.5 mg/L | 3.9 | 3.4 | 3.6 |
| 10. | Amylase (U/L) | 0–140 U/L | 98 U\L | 68 U\L | 179 U\L |
| 11. | Lipase (U/L) | 0–60 U/L | 58 | 49 | 47 |
| 12. | Blood sodium level (mEq/L) | 135–145 mEq/L | 139 | 137 | 145 |
| 13. | Blood potassium level (mEq/L) | 3.6–5.2 mEq/L | 3.9 | 3.7 | 4.1 |
| 14. | Blood calcium level (mg/dL) | 8.5–10.5 mg/dL | 6.7 | 6.9 | 7.7 |
| 15. | Triglyceride (mg/dL) | 40–150 mg/dL | 210 | 200 | 219 |
| 16. | Blood urea nitrogen (mg/dL) | 8–20 mg/dL | 50 | 110 | 120 |
| 17. | Creatinine (mg/dL) | 0.5–1.2 mg/dL | 1.2 | 1.2 | 1.1 |
| S. No. . | Parameters . | Normal range . | On admission . | During laparotomy . | Post-operatively . |
|---|---|---|---|---|---|
| 1. | WBC count (109 cells/L) | 3.5–9.5 109 cells/L | 13 × 109 | 19 × 109 | 8.9 × 109 |
| 2. | Neutrophil (%) | 50–70% | 73 | 79 | 83 |
| 3. | Lymphocyte (%) | 20–40% | 16 | 17 | 18 |
| 4. | Haemoglobin g/dL) | 12–16 g/dL | 13.3 g/dL | 12.1 g/dL | 11.7 g/dL |
| 5. | Platelet count (109 cells/L) | 125–350 109 | 240 | 230 | 210 |
| 6. | Haematocrit (%) | 36–48% | 42 | 44 | 40 |
| 7. | AST (U/L) | 5–45 U/L | 123 | 127 | 149 |
| 8. | ALT (U/L) | 5–40 U/L | 97 | 111 | 102 |
| 9. | Albumin (g/L) | 3.5–5.5 mg/L | 3.9 | 3.4 | 3.6 |
| 10. | Amylase (U/L) | 0–140 U/L | 98 U\L | 68 U\L | 179 U\L |
| 11. | Lipase (U/L) | 0–60 U/L | 58 | 49 | 47 |
| 12. | Blood sodium level (mEq/L) | 135–145 mEq/L | 139 | 137 | 145 |
| 13. | Blood potassium level (mEq/L) | 3.6–5.2 mEq/L | 3.9 | 3.7 | 4.1 |
| 14. | Blood calcium level (mg/dL) | 8.5–10.5 mg/dL | 6.7 | 6.9 | 7.7 |
| 15. | Triglyceride (mg/dL) | 40–150 mg/dL | 210 | 200 | 219 |
| 16. | Blood urea nitrogen (mg/dL) | 8–20 mg/dL | 50 | 110 | 120 |
| 17. | Creatinine (mg/dL) | 0.5–1.2 mg/dL | 1.2 | 1.2 | 1.1 |
Despite aggressive fluid management, analgesics and intravenous antibiotics, the condition of the patient did not improve. The following day, patient developed abdominal distension, with two episodes of bilious vomiting. His laboratory parameters showed leukocytosis with total leucocyte count (TLC) raised to 19 000/cumm with neutrophilia (Table 1). Repeat ultrasonography of abdomen was done which revealed mild to moderate pericholecystic fluid collection in the perihepatic space. Ultrasound guided needle aspiration was performed which confirmed bilious fluid in the perihepatic space. Urgent contrast-enhanced CT scan was performed which confirmed collection of fluid in the perihepatic space suggesting GB peroration.
Emergency exploratory laparotomy was performed though standard midline incision over abdomen. Massive greenish bile stained fluid, ~400 ml was drained. Intraoperatively, GBP at the fundus was noted. Perforation 3.6 mm × 1.2 mm in and necrosis at fundus with congestion & edema was noted from which bile spillage was seen (Fig. 1). Cholecystectomy with thorough peritoneal toileting was done and the specimen of gallbladder (Fig. 2) was sent for histopathological examination. Post-operatively, the recovery period was uneventful. Drain was removed on fifth post-operative day and the patient was shifted to non-COVID ward 1 week later after suture removal. Histopathological report revealed GBP at the fundus on gross examination and ischemic necrosis of gallbladder mucosa on microscopic examination.
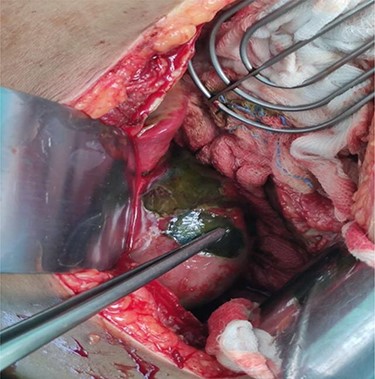
Intraoperative view of perforation at the fundus of gallbladder.
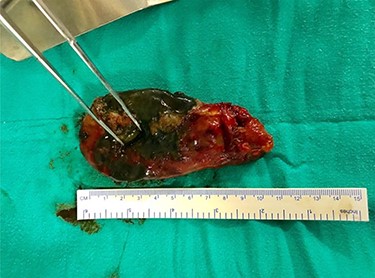
Gross specimen of gallbladder showing perforation in fundus region.
DISCUSSION
Perforation of the gallbladder is a life-threatening surgical problem owing to its relative infrequency, the difficulty of preoperative diagnosis and the associated high mortality [5–7]. The higher incidence of GBP are associated with calculi [8], with the incidence ranging upto 2%–11% of acute cholecystitis cases [9]. However, acalculous cholecystitis has been reported in patients with COVID-19, although it is unclear if this is due direct viral injury or due to the multiple confounding factors of general sepsis, prolonged parenteral nutrition or systemic cytokine release [8]. SARS-CoV-2 was not detected in the wall of the small intestine, appendix, gallbladder, bile and liver [10, 11]. This finding indicates towards a more complicated pathogenesis of gangrene of gallbladder than direct viral injury. Presence of thrombosis in medium sized vessels and dysregulated inflammatory response have been noted in gangrenous gallbladder of patients with COVID-19. This can be probable mechanism of injury to gallbladder [10]. Abdominal ultrasonography remains the first line of investigation; however, the sensitivity is variable and it is operator dependent. Contrast-enhanced computed tomography of the abdomen is the gold standard in identifying a perforated gallbladder with sensitivities ranging from 81.3% to 88.2%. Magnetic resonance cholangiopancreatography is similarly effective in the diagnosis of GBP [11].
Asti et al. reported three cases of gangrenous cholecystitis with perforation in patients with COVID-19 pneumonia during recovery period [11, 12]. Another case report also showed late presentation on Day 32 [10]. In contrast to these cases, our patient presented earlier in the course. Presentation of gangrenous cholecystitis was abdominal pain without features of peritonism in the case presenting on Day 32 [11]. Our patient presented in similar fashion. Asti et al. reported micro-perforation at fundus [12]. This points towards probable predilection towards fundus perforation in COVID-19 patients since the same site was perforated in our patient [13].
This case describes a young male COVID-19 with nonspecific abdominal symptoms. The basic laboratory investigations and the ultrasonography were unremarkable with no gallbladder calculi. This leads to a delay in the diagnosis till he developed features of sepsis and organ dysfunction. High degree of clinical suspicion and close monitoring in such patients is necessary to detect early deterioration.
CONCLUSION
COVID-19 is known to have GI manifestations. GBP is a rare manifestation of 2019 novel coronavirus. Patients can present during any period with features of acute abdomen, mostly without features of peritonism. The mechanism of pathogenesis is most probably thrombosis of medium sized vessels and dysregulated immune response.
ACKNOWLEDGEMENT
We would like to thank the patient and department of surgery, Shree Birendra Hospital, Chhauni, for their contribution to this case report.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



