-
PDF
- Split View
-
Views
-
Cite
Cite
Alfonso Scarpa, Arianna Di Stadio, Pietro De Luca, Matteo Calvanese, Pasquale Viola, Domenico Tassone, Luca de Campora, Matteo Simone, Giampietro Ricci, Daniela Messineo, Massimo Ralli, Claudia Cassandro, How can we manage Bezold abscess emergency in COVID-19 pandemic? Blind ceftriaxone and metronidazole treatment to avoid infection spread†, Journal of Surgical Case Reports, Volume 2021, Issue 10, October 2021, rjab493, https://doi.org/10.1093/jscr/rjab493
Close - Share Icon Share
Abstract
Bezold abscess (BA) can be a rare complication of different forms of otitis media. We describe a rare case of BA determined by Escherichia Coli. Because of COVID-19 restriction, the surgery had to be delayed up to the swab results. To avoid infection spread, the patient was treated by blind antibiotic treatment until the surgical drainage of mastoid and neck. Thanks to the treatment with broad-spectrum antibiotics, the progression and the spread of the infection during COVID-19 investigation was avoided. Delayed surgery could expose the patient to the risk of severe brain infection caused by the E. Coli.
INTRODUCTION
Bezold abscess (BA) can be a rare complication of acute, chronic or cholesteatomatous otitis media [1]; the infection can be determined by several pathogens, e.g. Streptococcus [2]. Some of these pathogens can be more dangerous than others [2] and the infection has to be immediately treated to avoid risk of spreading in the surrounding tissues [1–8].
| Author . | Year . | Number of cases . | Sex . | Age . | Culture . | Remarks . |
|---|---|---|---|---|---|---|
| Lyoubi et al. | 2020 | 1 | M | 62 | Polymicrobial | / |
| Rodrigues Silva et al. | 2020 | 1 | M | 67 | Polymicrobial | Necrotizing fasciitis of the shoulder with extension to the chest |
| Polony et al. | 2020 | 1 | F | 76 | / | Cholesteatoma, left sigmoid sinus and jugular bulb thrombosis |
| Lindquist et al. | 2020 | 1 | F | 66 | / | Advanced cervical cancer on chemotherapy, temporal bone paraganglioma, cholesteatoma and necrotizing fascitis |
| Malik et al. | 2019 | 1 | M | 55 | Staphylococcus epidermidis | Osteomyelitis |
| Al-Zahid et al. | 2019 | 1 | M | 49 | / | / |
| Eswaran et al. | 2019 | 1 | F | 15 | Mycobacterium tuberculosis | TBC |
| Yaita et al. | 2018 | 1 | F | 70 | Streptococcus constellatus | Lemierre’s syndrome |
| Mustafa et al. | 2018 | 1 | F | 14 | Streptococcus pneumoniae | Right lateral sinus thrombosis |
| Syngh et al. | 2018 | 1 | M | 38 | M. tuberculosis | TBC |
| Nasir et al. | 2017 | 1 | M | 52 | Klebsiella pneumoniae | / |
| Zer Toros et al. | 2017 | 1 | M | 45 | S. constellatus | Left cholesteatoma associated with ipsilateral lateral sinus thrombophlebitis and meningitis |
| Katayama et al. | 2017 | 1 | M | 52 | S. pneumoniae | / |
| Quoraishi et al. | 2017 | 1 | M | 44 | No growth | Citelli’s abscess |
| Lin et al. | 2015 | 1 | M | 49 | K. pneumoniae | / |
| Pradhananga et al. | 2014 | 1 | F | 14 | / | Cholesteatoma |
| Mantsopoulos et al. | 2015 | 1 | / | / | / | / |
| Nelson et al. | 2013 | 1 | F | 12 | Streptococcus pyogenes | / |
| Nawas et al. | 2013 | 1 | M | 74 | Pseudomonas aeruginosa | / |
| Lionello et al. | 2013 | 1 | M | 35 | Negative | |
| Li et al. | 2012 | 1 | F | 32 | / | Cholesteatoma |
| Janardhan et al. | 2012 | 1 | M | 60 | / | Cholesteatoma |
| Secko et al. | 2012 | 1 | M | 32 | / | HIV |
| Vlastos et al. | 2012 | 1 | F | 3 | / | Osteomyelitis |
| Sheikh et al. | 2011 | 1 | M | 26 | Ziehl–Neelsen stain-positive | / |
| Mascarinas et al. | 2010 | 1 | F | 77 | Streptococcus viridans | Prior soft palate squamous cell carcinoma and chemo and radiation therapies |
| Patel et al. | 2010 | 1 | M | 35 | / | HIV |
| McMullan et al. | 2009 | 1 | M | 8 | No growth | / |
| Ching et al. | 2006 | 1 | M | 14 | Streptococcus milleri | Post-streptococcal glomerulonephritis |
| Schöndorf et al. | 2004 | 1 | F | 10 weeks | Negative | / |
| Stokroos et al. | 2003 | 1 | M | 56 | / | / |
| Uchida et al. | 2002 | 1 | M | 25 | Polymicrobial | Cholesteatoma |
| Zapanta et al. | 2001 | 1 | F | 17 | Alfa-hemolytic streptococci | Multiple dural sinus thromboses |
| Marioni et al. | 2001 | 1 | M | 18 months | / | / |
| Spiegel et al. | 1998 | 3 | M | 55 | S. pneumoniae | Intravenous drug user |
| M | 33 | Polymicrobial | HIV | |||
| F | 27 | Negative | Intravenous drug user | |||
| Osma et al. | 2000 | 4 | / | / | / | / |
| Goldstein et al. | 1998 | 1 | / | / | / | Parapharyngeal space abscess |
| Lubianca Neto et al. | 1997 | 1 | M | 7 | / | Cholesteatoma and lateral sinus thrombosis |
| Pearson et al. | 1994 | 1 | M | 37 | S. milleri | Lateral sinus thrombosis and jugular vein thrombosis |
| Gaffney et al. | 1991 | 1 | M | 42 | Sterile | Cholesteatoma |
| Smouha et al. | 1989 | 5 | F | 23 | Polymicrobial | Parapharyngeal space abscess |
| M | 79 | Polymicrobial | Cholesteatoma | |||
| M | 62 | P. aeruginosa | / | |||
| M | 52 | Polymicrobial | ||||
| F | 73 | Polymicrobial | ||||
| El-Kholy | 1989 | 1 | M | 74 | Proteus spp. | Cholesteatoma |
| et al. | ||||||
| Moisa et al. | 1987 | 1 | M | 60 | Polymicrobial | Cholesteatoma |
| Moloy et al. | 1982 | 1 | M | 15 | Polymicrobial | / |
| Edison | 1980 | 1 | M | 43 | K. pneumoniae | / |
| Hill | 1968 | 1 | F | 2 | / | / |
| Tembe | 1965 | 1 | M | 23 | / | / |
| O’Malley | 1924 | 1 | M | 25 | / | / |
| Ouston | 1898 | 1 | F | 15 | / | / |
| Author . | Year . | Number of cases . | Sex . | Age . | Culture . | Remarks . |
|---|---|---|---|---|---|---|
| Lyoubi et al. | 2020 | 1 | M | 62 | Polymicrobial | / |
| Rodrigues Silva et al. | 2020 | 1 | M | 67 | Polymicrobial | Necrotizing fasciitis of the shoulder with extension to the chest |
| Polony et al. | 2020 | 1 | F | 76 | / | Cholesteatoma, left sigmoid sinus and jugular bulb thrombosis |
| Lindquist et al. | 2020 | 1 | F | 66 | / | Advanced cervical cancer on chemotherapy, temporal bone paraganglioma, cholesteatoma and necrotizing fascitis |
| Malik et al. | 2019 | 1 | M | 55 | Staphylococcus epidermidis | Osteomyelitis |
| Al-Zahid et al. | 2019 | 1 | M | 49 | / | / |
| Eswaran et al. | 2019 | 1 | F | 15 | Mycobacterium tuberculosis | TBC |
| Yaita et al. | 2018 | 1 | F | 70 | Streptococcus constellatus | Lemierre’s syndrome |
| Mustafa et al. | 2018 | 1 | F | 14 | Streptococcus pneumoniae | Right lateral sinus thrombosis |
| Syngh et al. | 2018 | 1 | M | 38 | M. tuberculosis | TBC |
| Nasir et al. | 2017 | 1 | M | 52 | Klebsiella pneumoniae | / |
| Zer Toros et al. | 2017 | 1 | M | 45 | S. constellatus | Left cholesteatoma associated with ipsilateral lateral sinus thrombophlebitis and meningitis |
| Katayama et al. | 2017 | 1 | M | 52 | S. pneumoniae | / |
| Quoraishi et al. | 2017 | 1 | M | 44 | No growth | Citelli’s abscess |
| Lin et al. | 2015 | 1 | M | 49 | K. pneumoniae | / |
| Pradhananga et al. | 2014 | 1 | F | 14 | / | Cholesteatoma |
| Mantsopoulos et al. | 2015 | 1 | / | / | / | / |
| Nelson et al. | 2013 | 1 | F | 12 | Streptococcus pyogenes | / |
| Nawas et al. | 2013 | 1 | M | 74 | Pseudomonas aeruginosa | / |
| Lionello et al. | 2013 | 1 | M | 35 | Negative | |
| Li et al. | 2012 | 1 | F | 32 | / | Cholesteatoma |
| Janardhan et al. | 2012 | 1 | M | 60 | / | Cholesteatoma |
| Secko et al. | 2012 | 1 | M | 32 | / | HIV |
| Vlastos et al. | 2012 | 1 | F | 3 | / | Osteomyelitis |
| Sheikh et al. | 2011 | 1 | M | 26 | Ziehl–Neelsen stain-positive | / |
| Mascarinas et al. | 2010 | 1 | F | 77 | Streptococcus viridans | Prior soft palate squamous cell carcinoma and chemo and radiation therapies |
| Patel et al. | 2010 | 1 | M | 35 | / | HIV |
| McMullan et al. | 2009 | 1 | M | 8 | No growth | / |
| Ching et al. | 2006 | 1 | M | 14 | Streptococcus milleri | Post-streptococcal glomerulonephritis |
| Schöndorf et al. | 2004 | 1 | F | 10 weeks | Negative | / |
| Stokroos et al. | 2003 | 1 | M | 56 | / | / |
| Uchida et al. | 2002 | 1 | M | 25 | Polymicrobial | Cholesteatoma |
| Zapanta et al. | 2001 | 1 | F | 17 | Alfa-hemolytic streptococci | Multiple dural sinus thromboses |
| Marioni et al. | 2001 | 1 | M | 18 months | / | / |
| Spiegel et al. | 1998 | 3 | M | 55 | S. pneumoniae | Intravenous drug user |
| M | 33 | Polymicrobial | HIV | |||
| F | 27 | Negative | Intravenous drug user | |||
| Osma et al. | 2000 | 4 | / | / | / | / |
| Goldstein et al. | 1998 | 1 | / | / | / | Parapharyngeal space abscess |
| Lubianca Neto et al. | 1997 | 1 | M | 7 | / | Cholesteatoma and lateral sinus thrombosis |
| Pearson et al. | 1994 | 1 | M | 37 | S. milleri | Lateral sinus thrombosis and jugular vein thrombosis |
| Gaffney et al. | 1991 | 1 | M | 42 | Sterile | Cholesteatoma |
| Smouha et al. | 1989 | 5 | F | 23 | Polymicrobial | Parapharyngeal space abscess |
| M | 79 | Polymicrobial | Cholesteatoma | |||
| M | 62 | P. aeruginosa | / | |||
| M | 52 | Polymicrobial | ||||
| F | 73 | Polymicrobial | ||||
| El-Kholy | 1989 | 1 | M | 74 | Proteus spp. | Cholesteatoma |
| et al. | ||||||
| Moisa et al. | 1987 | 1 | M | 60 | Polymicrobial | Cholesteatoma |
| Moloy et al. | 1982 | 1 | M | 15 | Polymicrobial | / |
| Edison | 1980 | 1 | M | 43 | K. pneumoniae | / |
| Hill | 1968 | 1 | F | 2 | / | / |
| Tembe | 1965 | 1 | M | 23 | / | / |
| O’Malley | 1924 | 1 | M | 25 | / | / |
| Ouston | 1898 | 1 | F | 15 | / | / |
| Author . | Year . | Number of cases . | Sex . | Age . | Culture . | Remarks . |
|---|---|---|---|---|---|---|
| Lyoubi et al. | 2020 | 1 | M | 62 | Polymicrobial | / |
| Rodrigues Silva et al. | 2020 | 1 | M | 67 | Polymicrobial | Necrotizing fasciitis of the shoulder with extension to the chest |
| Polony et al. | 2020 | 1 | F | 76 | / | Cholesteatoma, left sigmoid sinus and jugular bulb thrombosis |
| Lindquist et al. | 2020 | 1 | F | 66 | / | Advanced cervical cancer on chemotherapy, temporal bone paraganglioma, cholesteatoma and necrotizing fascitis |
| Malik et al. | 2019 | 1 | M | 55 | Staphylococcus epidermidis | Osteomyelitis |
| Al-Zahid et al. | 2019 | 1 | M | 49 | / | / |
| Eswaran et al. | 2019 | 1 | F | 15 | Mycobacterium tuberculosis | TBC |
| Yaita et al. | 2018 | 1 | F | 70 | Streptococcus constellatus | Lemierre’s syndrome |
| Mustafa et al. | 2018 | 1 | F | 14 | Streptococcus pneumoniae | Right lateral sinus thrombosis |
| Syngh et al. | 2018 | 1 | M | 38 | M. tuberculosis | TBC |
| Nasir et al. | 2017 | 1 | M | 52 | Klebsiella pneumoniae | / |
| Zer Toros et al. | 2017 | 1 | M | 45 | S. constellatus | Left cholesteatoma associated with ipsilateral lateral sinus thrombophlebitis and meningitis |
| Katayama et al. | 2017 | 1 | M | 52 | S. pneumoniae | / |
| Quoraishi et al. | 2017 | 1 | M | 44 | No growth | Citelli’s abscess |
| Lin et al. | 2015 | 1 | M | 49 | K. pneumoniae | / |
| Pradhananga et al. | 2014 | 1 | F | 14 | / | Cholesteatoma |
| Mantsopoulos et al. | 2015 | 1 | / | / | / | / |
| Nelson et al. | 2013 | 1 | F | 12 | Streptococcus pyogenes | / |
| Nawas et al. | 2013 | 1 | M | 74 | Pseudomonas aeruginosa | / |
| Lionello et al. | 2013 | 1 | M | 35 | Negative | |
| Li et al. | 2012 | 1 | F | 32 | / | Cholesteatoma |
| Janardhan et al. | 2012 | 1 | M | 60 | / | Cholesteatoma |
| Secko et al. | 2012 | 1 | M | 32 | / | HIV |
| Vlastos et al. | 2012 | 1 | F | 3 | / | Osteomyelitis |
| Sheikh et al. | 2011 | 1 | M | 26 | Ziehl–Neelsen stain-positive | / |
| Mascarinas et al. | 2010 | 1 | F | 77 | Streptococcus viridans | Prior soft palate squamous cell carcinoma and chemo and radiation therapies |
| Patel et al. | 2010 | 1 | M | 35 | / | HIV |
| McMullan et al. | 2009 | 1 | M | 8 | No growth | / |
| Ching et al. | 2006 | 1 | M | 14 | Streptococcus milleri | Post-streptococcal glomerulonephritis |
| Schöndorf et al. | 2004 | 1 | F | 10 weeks | Negative | / |
| Stokroos et al. | 2003 | 1 | M | 56 | / | / |
| Uchida et al. | 2002 | 1 | M | 25 | Polymicrobial | Cholesteatoma |
| Zapanta et al. | 2001 | 1 | F | 17 | Alfa-hemolytic streptococci | Multiple dural sinus thromboses |
| Marioni et al. | 2001 | 1 | M | 18 months | / | / |
| Spiegel et al. | 1998 | 3 | M | 55 | S. pneumoniae | Intravenous drug user |
| M | 33 | Polymicrobial | HIV | |||
| F | 27 | Negative | Intravenous drug user | |||
| Osma et al. | 2000 | 4 | / | / | / | / |
| Goldstein et al. | 1998 | 1 | / | / | / | Parapharyngeal space abscess |
| Lubianca Neto et al. | 1997 | 1 | M | 7 | / | Cholesteatoma and lateral sinus thrombosis |
| Pearson et al. | 1994 | 1 | M | 37 | S. milleri | Lateral sinus thrombosis and jugular vein thrombosis |
| Gaffney et al. | 1991 | 1 | M | 42 | Sterile | Cholesteatoma |
| Smouha et al. | 1989 | 5 | F | 23 | Polymicrobial | Parapharyngeal space abscess |
| M | 79 | Polymicrobial | Cholesteatoma | |||
| M | 62 | P. aeruginosa | / | |||
| M | 52 | Polymicrobial | ||||
| F | 73 | Polymicrobial | ||||
| El-Kholy | 1989 | 1 | M | 74 | Proteus spp. | Cholesteatoma |
| et al. | ||||||
| Moisa et al. | 1987 | 1 | M | 60 | Polymicrobial | Cholesteatoma |
| Moloy et al. | 1982 | 1 | M | 15 | Polymicrobial | / |
| Edison | 1980 | 1 | M | 43 | K. pneumoniae | / |
| Hill | 1968 | 1 | F | 2 | / | / |
| Tembe | 1965 | 1 | M | 23 | / | / |
| O’Malley | 1924 | 1 | M | 25 | / | / |
| Ouston | 1898 | 1 | F | 15 | / | / |
| Author . | Year . | Number of cases . | Sex . | Age . | Culture . | Remarks . |
|---|---|---|---|---|---|---|
| Lyoubi et al. | 2020 | 1 | M | 62 | Polymicrobial | / |
| Rodrigues Silva et al. | 2020 | 1 | M | 67 | Polymicrobial | Necrotizing fasciitis of the shoulder with extension to the chest |
| Polony et al. | 2020 | 1 | F | 76 | / | Cholesteatoma, left sigmoid sinus and jugular bulb thrombosis |
| Lindquist et al. | 2020 | 1 | F | 66 | / | Advanced cervical cancer on chemotherapy, temporal bone paraganglioma, cholesteatoma and necrotizing fascitis |
| Malik et al. | 2019 | 1 | M | 55 | Staphylococcus epidermidis | Osteomyelitis |
| Al-Zahid et al. | 2019 | 1 | M | 49 | / | / |
| Eswaran et al. | 2019 | 1 | F | 15 | Mycobacterium tuberculosis | TBC |
| Yaita et al. | 2018 | 1 | F | 70 | Streptococcus constellatus | Lemierre’s syndrome |
| Mustafa et al. | 2018 | 1 | F | 14 | Streptococcus pneumoniae | Right lateral sinus thrombosis |
| Syngh et al. | 2018 | 1 | M | 38 | M. tuberculosis | TBC |
| Nasir et al. | 2017 | 1 | M | 52 | Klebsiella pneumoniae | / |
| Zer Toros et al. | 2017 | 1 | M | 45 | S. constellatus | Left cholesteatoma associated with ipsilateral lateral sinus thrombophlebitis and meningitis |
| Katayama et al. | 2017 | 1 | M | 52 | S. pneumoniae | / |
| Quoraishi et al. | 2017 | 1 | M | 44 | No growth | Citelli’s abscess |
| Lin et al. | 2015 | 1 | M | 49 | K. pneumoniae | / |
| Pradhananga et al. | 2014 | 1 | F | 14 | / | Cholesteatoma |
| Mantsopoulos et al. | 2015 | 1 | / | / | / | / |
| Nelson et al. | 2013 | 1 | F | 12 | Streptococcus pyogenes | / |
| Nawas et al. | 2013 | 1 | M | 74 | Pseudomonas aeruginosa | / |
| Lionello et al. | 2013 | 1 | M | 35 | Negative | |
| Li et al. | 2012 | 1 | F | 32 | / | Cholesteatoma |
| Janardhan et al. | 2012 | 1 | M | 60 | / | Cholesteatoma |
| Secko et al. | 2012 | 1 | M | 32 | / | HIV |
| Vlastos et al. | 2012 | 1 | F | 3 | / | Osteomyelitis |
| Sheikh et al. | 2011 | 1 | M | 26 | Ziehl–Neelsen stain-positive | / |
| Mascarinas et al. | 2010 | 1 | F | 77 | Streptococcus viridans | Prior soft palate squamous cell carcinoma and chemo and radiation therapies |
| Patel et al. | 2010 | 1 | M | 35 | / | HIV |
| McMullan et al. | 2009 | 1 | M | 8 | No growth | / |
| Ching et al. | 2006 | 1 | M | 14 | Streptococcus milleri | Post-streptococcal glomerulonephritis |
| Schöndorf et al. | 2004 | 1 | F | 10 weeks | Negative | / |
| Stokroos et al. | 2003 | 1 | M | 56 | / | / |
| Uchida et al. | 2002 | 1 | M | 25 | Polymicrobial | Cholesteatoma |
| Zapanta et al. | 2001 | 1 | F | 17 | Alfa-hemolytic streptococci | Multiple dural sinus thromboses |
| Marioni et al. | 2001 | 1 | M | 18 months | / | / |
| Spiegel et al. | 1998 | 3 | M | 55 | S. pneumoniae | Intravenous drug user |
| M | 33 | Polymicrobial | HIV | |||
| F | 27 | Negative | Intravenous drug user | |||
| Osma et al. | 2000 | 4 | / | / | / | / |
| Goldstein et al. | 1998 | 1 | / | / | / | Parapharyngeal space abscess |
| Lubianca Neto et al. | 1997 | 1 | M | 7 | / | Cholesteatoma and lateral sinus thrombosis |
| Pearson et al. | 1994 | 1 | M | 37 | S. milleri | Lateral sinus thrombosis and jugular vein thrombosis |
| Gaffney et al. | 1991 | 1 | M | 42 | Sterile | Cholesteatoma |
| Smouha et al. | 1989 | 5 | F | 23 | Polymicrobial | Parapharyngeal space abscess |
| M | 79 | Polymicrobial | Cholesteatoma | |||
| M | 62 | P. aeruginosa | / | |||
| M | 52 | Polymicrobial | ||||
| F | 73 | Polymicrobial | ||||
| El-Kholy | 1989 | 1 | M | 74 | Proteus spp. | Cholesteatoma |
| et al. | ||||||
| Moisa et al. | 1987 | 1 | M | 60 | Polymicrobial | Cholesteatoma |
| Moloy et al. | 1982 | 1 | M | 15 | Polymicrobial | / |
| Edison | 1980 | 1 | M | 43 | K. pneumoniae | / |
| Hill | 1968 | 1 | F | 2 | / | / |
| Tembe | 1965 | 1 | M | 23 | / | / |
| O’Malley | 1924 | 1 | M | 25 | / | / |
| Ouston | 1898 | 1 | F | 15 | / | / |
Very few cases of BA have been described in the literature (Table 1) and, in none of these, the presence of Escherichia Coli in the BA was specifically found; E. Coli can be very harmful if not promptly and adequately treated and, especially during COVID-19 era, in which all surgical procedures are slightly delayed after the result of COVID-19 swab, the delay in treating the bacterial infection may expose patients to the risk of worsening the condition. Blind antibiotic treatment is generally discouraged due to the risk of resistance; however, in COVID-19 pandemic, blind combined antibiotic therapy could be necessary.
We describe the management of a BA due to E. coli in a patient affected by cholesteatomatous chronic otitis media (CCOM) during the COVID-19 pandemic.
CASE REPORT
A 17-year-old boy with cognitive-emotional impairment presented in August 2020, to our emergency department, complaining about the onset of a left-sided otalgia with purulent otorrhea, neck stiffness and pain on the ipsilateral side without fever. He had a history of bilateral CCOM for 8 years with recurrent flare-up, which was periodically treated by systemic antibiotic therapy and ear washing with acetic acid due to patient’s refusal of surgery. The patient presented stiffness of the left side of the neck with hot and sweaty skin, associated with hyperemia and swelling in the left latero-cervical region. The inflammation affected the left mastoid region and extended caudally into the left supraclavicular fossa (Fig. 1A).
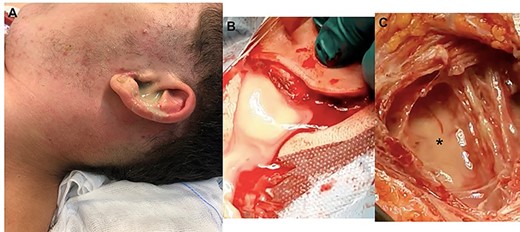
Pre- and intra-operatory images; (A) purulent secretion from the external left canal associated with swelling of retro-auricular area (mastoid) and neck; the skin is red as a sign of inflammation; (B) after retro-auricular incision and press on the neck, the purulent secretion comes out; (C) a curettage of the neck area is necessary to remove the residual secretion (black asterisk) which has not been expelled through the retro-auricular incision.
The otoscopic examination revealed bilateral perforation of the tympanic membrane and the presence of purulent discharge in the left ear (Fig. 1B). Nasal endoscopy identified II grade adenoid hypertrophy with complete obliteration of left choanal opening and tubal ostium (Fig. 2A and B). A pure tone audiometry, performed only through bone conduction, showed bilaterally normal auditory threshold. Neck ultrasound identified the presence of abundant fluid in the left latero-cervical area, which extended from the retro-auricular region up to the ipsilateral supraclavicular area.
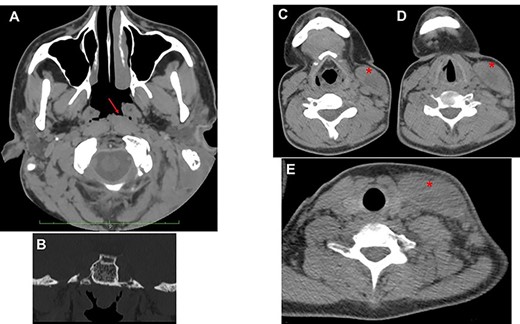
(A) axial view; the red arrow shows edematous torus tubarius; compared to contralateral, the adenoid is hypertrophic but median; the presence of both these conditions contributes to ostium obstruction of the left tuba (B); (C–E) from top to down, the red asterisk shows the imbibition of sternocleidomastoid muscle sign of BA.
Head and neck contrast-enhanced computed tomography (CT) scan confirmed the presence of the fluid below the left sternocleidomastoid muscle (Fig. 2C–E) with massive opacification of the mastoid cells and the middle ear (Fig. 3A). Inflammatory tissue was identified in the middle ear with erosion of the ossicular bone chain (Fig. 3B). A diagnosis of CCOM with BA was made.
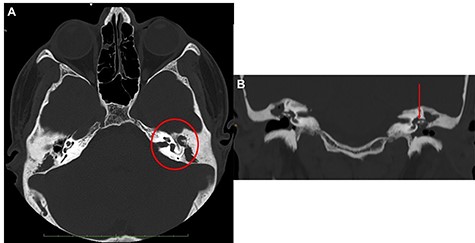
Ear CT scan; (A) axial view; the red circle shows the middle ear filled with inflammatory tissue, with erosion of the ossicular chain; (B) coronal view (the red arrow shows the erosion of malleus and incus); the malleus is not lateralized.
Intravenous therapy with ceftriaxone (2 g every 24 h), metronidazole (500 mg every 8 h), paracetamol (1 g every 8 h) and pantoprazole (40 mg every 24 h) was started because, due to COVID-19 restriction, it was not possible to immediately perform surgery before confirmation of negative COVID-19 swab. Once received the results of the COVID-19 test, we performed mastoidectomy, drainage of neck abscess (Fig. 1C), adenoidectomy and inspection of middle ear.
The purulent secretion was collected from external ear before starting surgery and then from the neck (Fig. 1) after a small incision of the skin and from middle ear during its inspection; all samples were sent to the microbiologist for culture and antibiogram.
The inspection of the middle ear revealed the erosion of the ossicular chain without lateralization of the malleus, and presence of keratinizing squamous epithelium, confirming the CT findings.
Escherichia coli was present in all three samples of the purulent secretion, and the antibiogram confirmed that the microbe was sensitive to the previously administered antibiotic therapy (ceftriaxone and metronidazole), although the dosage was insufficient to solve the infection. Due to the persistence of the infection after the 2 days of treatment, antibiotic therapy was changed increasing ceftriaxone (2 g/12 h) and metronidazole (500 mm/6 h). The treatment was continued for 7 days until patient discharge 10 days after admission.
In October 2020, the patient underwent left tympanoplasty to remove the inflammatory tissue and cholesteatoma and to perform an ossiculoplasty. The last follow-up (January 2021) showed cleanness of the left external auditory canal, good results of tympanoplasty, normal bilateral auditory thresholds and clean neck area bilaterally.
DISCUSSION
To the best of our knowledge, the case reports presented in the literature [1–4] have never clearly described the presence of E. Coli as pathogen responsible of BA.
Today, thanks to the use of antibiotics, BA has become a rare entity. The infection is generally caused by a series of microbes, which in the most of the cases, are easy treatable by a prompt antibiotic therapy. In fact, despite the fact that BA infections are often caused by polymicrobial flora, the Streptococcus pneumoniae is the most common microbe identified. Followed by less common pathogens, such as Pseudomonas aeruginosa, Klebsiella pneumoniae and Mycobacterium tuberculosis, and rare entities, such as Staphylococcus epidermidis and Proteus mirabilis [1–8].
Unlike these pathogens, E. coli, which is commonly present in the gut and has immune-stimulating capacity [9], can be very harmful because resistant to antimicrobial treatments.
The delay of the surgery, due to the restrictions during COVID-19 pandemic, and the impossibility of the analysis of purulent secretion could expose the patient to severe risk of brain infection. An early treatment by broad-spectrum antibiotics, despite not being sufficient to solve the infection (dosage too low to eradicate E. Coli), was sufficient to avoid the progression of the infection until the surgical curettage of the BA. After surgery, the antibiotic treatment was properly modified to reach complete eradication of the pathogen without patient presented short- and long-term complication.
CONCLUSION
COVID-19 pandemic is having an important impact on the management of many conditions, and the impact on the patients’ health can be more relevant than expected. In the present case, the absence of an immediate surgical drainage of BA, associated with the incapacity of performing the analysis of purulent secretion in emergency, could transform a quite low-risk condition into a life-threatening disease if not promptly and correctly managed.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
The study has been approved by the International Review Board of the hospital without release of ID in respect of law country for these types of studies.
References
Mustafa A, Toçi B, Thaçi H, Gjikolli B, Baftiu N.
Nawas MT, Daruwalla VJ, Spirer D, Micco AG, Nemeth AJ.
Silva VAR, Almeida AS, Lavinsky J, Pauna HF, Castilho AM, Chone CT, et al.
Author notes
A. Scarpa and A. Di Stadio contributed equally to this article.



