-
PDF
- Split View
-
Views
-
Cite
Cite
Daisuke Nakamura, Masayuki Toishi, Takao Sakaizawa, Sachie Koike, Hideki Nishimura, Postoperative chylothorax following lung cancer surgery with an aberrant course of thoracic duct: a case report, Journal of Surgical Case Reports, Volume 2021, Issue 10, October 2021, rjab490, https://doi.org/10.1093/jscr/rjab490
Close - Share Icon Share
Abstract
Postoperative chylothorax occurs relatively rarely after pulmonary resections, often caused intraoperatively by injury to the thoracic duct. We describe a case of postoperative chylothorax after lung cancer surgery with an aberrant thoracic duct course. A 66-year-old man showed abnormal findings on chest computed tomography (CT) during health screening and was suspected with primary lung cancer. Then, he underwent a right upper lobectomy with mediastinal lymph-node dissection. The histopathological findings confirmed lung adenocarcinoma. However, the patient developed a postoperative chylothorax and underwent revision surgery. An abnormally running thoracic duct, which was expected to flow into the right venous angle, was found at the cranial side of the right superior mediastinal dissection area and was clipped. Considering the many variations in the route of the thoracic duct, thoracic surgeons should remain alert for postoperative chylothorax when performing lung cancer surgery with mediastinal lymph-node dissection and prepare treatment strategies accordingly.
INTRODUCTION
Postoperative chylothorax is a relatively rare complication following pulmonary resection with an incidence rate from 1.4 to 2.3%. It occurs after pulmonary resection with lymph-node dissection [1, 2] and is primarily caused intraoperatively by injury to the thoracic duct; however, the thoracic duct has many anatomical variations [3, 4]. Herein, we report a case of postoperative chylothorax with an aberrant course of the thoracic duct after right upper lobectomy with mediastinal lymph-node dissection.
CASE
A 66-year-old man with a history of diabetes mellitus and atrial fibrillation visited our hospital after a routine health screening when an abnormality was detected on chest computed tomography (CT). His physical examination was unremarkable. A tumor biomarker test revealed elevated carcinoembryonic antigen (7.3 ng/ml) levels; all other biochemical markers were within normal limits. The chest CT revealed an irregular emphysematous cyst and an infiltrative shadow, 60 mm in diameter, along the cyst wall in the right upper lobe (Fig. 1a and b). The hilar or mediastinal lymph nodes were not swollen. We made a preoperative diagnosis of suspected primary lung cancer (cT3N0M0, stage IIB) and performed right upper lobectomy with hilar and mediastinal lymph-node dissection by video-assisted thoracic surgery. Intraoperatively, the mediastinal pleura was incised in the right superior mediastinal dissection using an energy device, and the anterior surface of the trachea was dissected with clipping, as appropriate. Retrospectively, a tortuous duct was detected near the cranial side in the superior mediastinal dissection area, which was dissected using the energy device (Fig. 2). From the histological findings, lung adenocarcinoma (pT1cN0M0, stageIA3) was diagnosed. On postoperative day (POD) 1, milky fluid was drained after the patient resumed eating. The triglyceride level in the pleural fluid was elevated to 457 mg/dl, and the patient was diagnosed with chylothorax. A low-fat diet (fat intake 35 g/day) was administered to the patient, but 500-ml fluid drainage was observed in 24 h. A revision surgery was performed on POD 2, 3 h after intake of 10-g butter and 200-ml milk. A disconnected duct was observed in the cranial side of the right superior mediastinal dissection area from which the chyle leakage originated. The disconnected duct coursed from the anterior mediastinum to the ventral side of the superior vena cava. This was suggested as the main trunk of the thoracic duct flowing into the right venous angle and the cause of the chylothorax. The thoracic duct could not be identified in the right posterior mediastinum; therefore, the thoracic duct was clipped in the right superior mediastinal dissection area (Supplementary Video 1). After reoperation, no chyle leakage occurred, and the patient’s postoperative course was uneventful. At 18 months postoperatively, there was no recurrence of either lung cancer or chylothorax. The patient provided informed consent for publication of this case report.
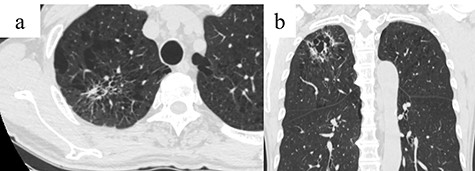
Chest CT showing an infiltrative shadow along the cyst wall with an irregular emphysematous cyst in the right upper lobe (arrow); (a) Axial view and (b) coronal view.
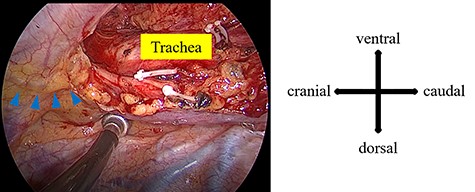
During the first surgery, a tortuous duct was detected in the cranial side of the superior mediastinal dissection area (blue arrowhead).
DISCUSSION
Postoperative chylothorax is a rare complication with an incidence of 1.4–2.3% after lung resection with lymph-node dissection [1, 2]. The thoracic duct usually courses posteriorly between the azygos vein and the aorta in the right thorax and crosses to the left side at the level of the fifth thoracic vertebra. It finally flows into the subclavian vein and the left venous angle. However, the thoracic duct may have anatomical variations, with incidences reported to range between 13.7 and 60% [3–5]. Minegishi et al. reported bilateral chylothorax after lung cancer surgery via median sternotomy with a lymphatic network in the anterior and middle mediastinum [6]. Rouiller et al. reported a case of chylothorax with bilateral thoracic duct ligation [7]. Still, the inflow of the thoracic duct into the right venous angle is rare, with the incidence rates reported as 0.82–4.1% [3, 4].
For our patient, we speculated that the thoracic duct probably ascended to the left side of the vertebral body, crossed to the right side and entered the right venous angle, as there was no thoracic duct in the right posterior mediastinum. Okuda et al. reported six classifications of the anatomical thoracic duct course based on magnetic resonance-thoracic ductography [3]. According to Okuda et al.’s classification, our case was presumed to be of type VII or VIII. The cranial side of the right superior mediastinal dissection is a less common site for clipping and ligation of the thoracic duct. However, the variations of the course of thoracic duct should be considered, and accordingly, clipping or ligation should be performed when ducts suspicious of the thoracic duct or lymphatic vessels are detected.
The treatments for postoperative chylothorax after lung cancer surgery include thoracic drainage, pleurodesis, low-fat diet, oral intake cessation with total parenteral nutrition, somatostatin, thoracic duct embolization and thoracic duct ligation [1, 2, 8]. Lymphangiography is performed to determine the exact location of the thoracic duct and has a therapeutic effect on postoperative chylothorax as well [8–10]. However, since reoperation was required immediately after the first surgery, we did not perform lymphangiography in our patient. Nevertheless, performing lymphangiography is important to detect the exact course of the thoracic duct and identify the point of chyle leakage. Therefore, as early reoperation was conducted on POD 2 in our case, the intrathoracic adhesions were mild, and dissection and intraoperative manipulation were relatively easily performed.
CONCLUSION
We report a case of postoperative chylothorax after lung cancer surgery with an aberrant thoracic duct course. Our case report demonstrates the importance of being aware of the variations in the route of the thoracic duct that thoracic surgeons should be cognizant of while performing lung cancer surgeries with mediastinal lymph-node dissection. Further, appropriate treatment strategies for postoperative chylothorax should be determined.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
References
- chylothorax
- tissue dissection
- drug administration routes
- repeat surgery
- cranium
- thoracic duct
- lymph nodes
- mediastinum
- thoracic surgery specialty
- lung volume reduction
- lung cancer
- lung adenocarcinoma
- chest ct
- right upper lung lobectomy
- histopathology tests
- fluid flow
- lung cancer surgery
- lung excision



