-
PDF
- Split View
-
Views
-
Cite
Cite
Erika P Brigmon, Mishel S Malik, Shazli N Malik, Alicia Logue, Primary pancreatic cancer of the jejunum: a case report and brief review of literature, Journal of Surgical Case Reports, Volume 2021, Issue 10, October 2021, rjab469, https://doi.org/10.1093/jscr/rjab469
Close - Share Icon Share
Abstract
The variable clinical presentation of heterotopic pancreatic (HP) tissue and its malignant transformation makes the diagnosis very challenging. These lesions are very rare, usually not suspected upon initial presentation and for the most part, are diagnosed incidentally on review of pathology following surgical resection. In this study, we are reporting an adenocarcinoma arising from jejunal heterotopic pancreatic tissue in a 59-year-old female and a brief review of previously reported cases.
CASE REPORT
A 59-year-old female presented to the emergency room complaining of severe mid-epigastric pain and nausea. She had worsening pain, reflux, early satiety, constipation, fatigue, weight loss and progressive inability to tolerate oral intake for 7 months prior to presentation. Last 4 weeks prior to presentation she was able to tolerate only liquid diet. Significant comorbidities included hypertension and anxiety.
After clinical evaluation, the patient underwent computed tomography (CT) of abdomen and pelvis with IV contrast, which revealed fluid-filled esophagus, dilated stomach and first part of the small intestine with a transition point in the mid jejunum (Fig. 1). Her proximal small bowel, stomach and esophagus were significantly distended. She was subsequently decompressed with a nasogastric tube. For further delineation of this transition point, a magnetic resonance imaging (MRI) was performed, which confirmed a narrowed lumen secondary to an intrinsic mass of the jejunum (Fig. 2). Later, she underwent exploratory laparotomy with an evident transition point found at 30 cm from the ligament of Treitz (LT) (Fig. 3). We performed a partial small bowel resection and side-to-side stapled anastomosis. The rest of the bowel appeared healthy; there was no evidence of serosal involvement or extension to surrounding loops of bowel. Multiple enlarged lymph nodes were identified in the mesentery.
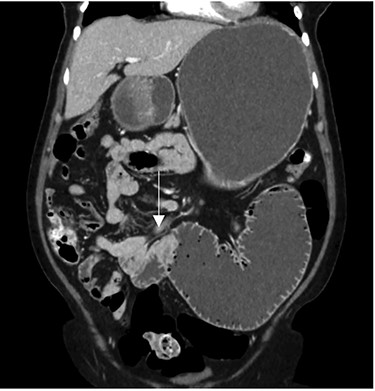
CT imaging—coronal view showing the transition point in the jejunum.
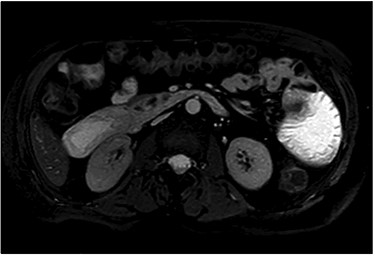
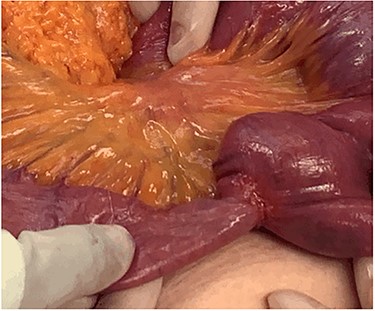
Stricturing lesion. The proximal small bowel was significantly dilated however, distally was viable and decompressed.
Macroscopically, the mass was ulcerated, well-defined 2.3 × 1.5 cm lesion present on the anti-mesenteric surface without serosal involvement and normal preservation of the remaining mucosal folds. (Fig. 4).
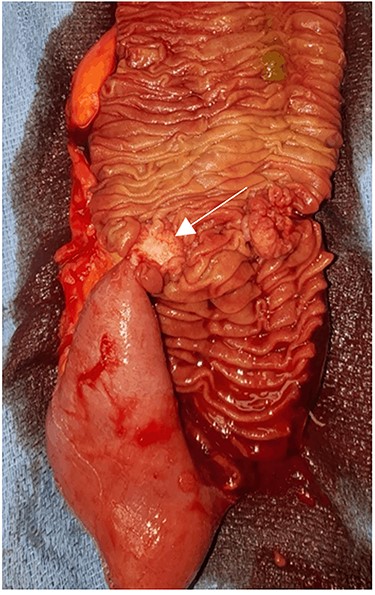
Small bowel lumen. The mucosa itself was relatively unremarkable in appearance with a tight fibrotic submucosal lesion (arrow).
Histologically, the lesion was identified as well-differentiated adenocarcinoma with mucinous features surrounded by desmoplastic stroma to poorly differentiated strands and dilated ducts with mucin pooling. The individual cells were cuboidal with high nuclear cytoplasmic ratio, irregular nuclear membranes, course nuclear chromatin and inconspicuous to prominent nucleoli (Fig. 5). Adjacent to the infiltrating carcinoma, foci of pancreatic ductal dysplasia (PAIN) were noted in direct communication with the benign pancreatic acini and ductal tissue which appeared typical of pancreatic heterotopia. The carcinoma infiltrated the entire thickness of the small bowel wall and infiltrated the subserosal adipose tissue. Multiple small deposits of tumor were also noted in the subserosal adipose tissue surrounded by desmoplastic reaction typical of pancreatic ductal cancers. Lymphovascular and perineural invasion were present. Seven out of 15 mesenteric lymph nodes were involved by metastatic carcinoma. The immunohistochemical stains were strongly positive for CK7 and Muc5 AC. No in situ lesion was present in the overlying jejunal mucosa. Therefore, it was clear that the carcinoma arose from the heterotopic pancreatic tissue present in the submucosa.
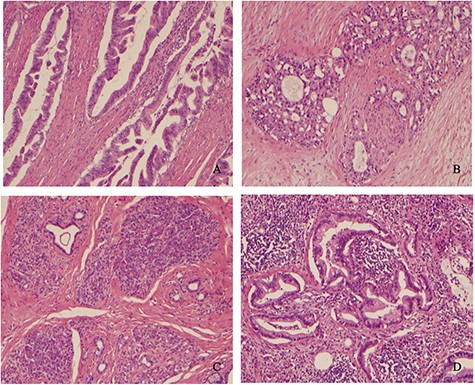
(A) Well-differentiated adenocarcinoma infiltrating muscularis propria. (B) Pancreatic adenocarcinoma showing perineural invasion. (C) Heterotopic benign pancreatic tissue, acini and ducts (Heinrich type 2) in the submucosa adjacent to PAIN and infiltrating cancer. (D) Carcinoma invading mesenteric lymph node.
The patient’s hospital course was uneventful, and she was discharged home after complete return of bowel function. The patient was evaluated postop in conjunction with the medical oncology team who planned on adjuvant chemotherapy.
DISCUSSION
A total of 14 cases of adenocarcinoma arising in Heterotopic pancreatic tissue in the jejunum have been reported in the world’s literature dating from 1988 to 2019 as shown in Table 1. In these reports, the patients’ ages ranged between 54 and 85 years, with the majority of cases in the sixth and seventh decade. The male to female ratio is 1 to 1. According to the Heinrich classification, six were Type 1, five were Type 2, and one was Type 3. Majority of the cancers were ductal adenocarcinomas, and one was acinar adenocarcinoma. The tumor size ranged between 1.5 and 4.7 cm. Short-term follow-up information was available in 6 out of 14 patients, 2 of whom died with metastasis within 5 months. Two are alive with metastases (liver 1 year, peritoneal carcinomatosis 9 months). Two are free of disease. Although a small number, the follow-up data underscores the severe nature of this malignancy and a need for aggressive management.
Reported cases of adenocarcinoma arising in heterotopic pancreatic tissue in the jejunum.
| Year . | Author . | Age . | Sex . | Distance from LT . | Symptoms . | Appearance . | Size . | Histology . | LNs mets . | Immunostains . | Heinrich . | Chemotherapy . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1988 | Persson et al | 85 | F | 100 cm | NA | NA | 1.5 cm | NA | N/A | NA | Type 1 | N/A | NA |
| 1990 | Fujiki et | 54 | M | 50 cm | NA | NA | 3 × 2 cm | NA | NA | NA | Type 1 | NA | NA |
| 1993 | Satoh et al | 64 | M | 110 cm | Abdominal pain | NA | 2 × 1.5 cm | Ductal adenocarcinoma | NA | NA | Type 1 | NA | NA |
| 1999 | Makhlouf et al | 71 | M | unknown | None | Central ulceration | 1.5 cm ulcer, 3.5 cm tumor | Acinar adenocarcinoma | None | CK7, alpha1-antitrypsin, alpha1-antichymotrypson, BER-EP4 and CEA | Type 1 | N/A | Liver metastasis (1 year) |
| 1999 | Makhlouf et al | 61 | M | 8 cm | Intestinal obstruction | Cauliflower-like mucosal lesion | 1.5 cm | Ductal adenocarcinoma | Present | NA | Type 1 | NA | NA |
| 1999 | Arao et al | 63 | M | 20 cm | Epigastric discomfort, vomiting | NA | 4 × 2 cm | Ductal adenocarcinoma | N/A | NA | Type 2 | N/A | NA |
| 2007 | Sato et al | 78 | F | 50 cm | Abdominal pain, nausea, vomiting | Yellowish-brown protruding tumor | 3 × 2 cm | Ductal adenocarcinoma | NA | NA | Type 2 | NA | Uneventful |
| 2008 | Fujita et al | 64 | F | NA | Distension, epigastric pain | Ulcerated tumor | 2 cm | NA | Present | NA | yes | Peritoneal carcinomatsis. Death 5 months. after diagnosis. | |
| 2010 | Fujiwara et al | 76 | F | NA | Post pandrial vomiting | 2 × 1.5 cm | Ductal adenocarcinoma | Present | NA | Type 3 | N/A | Liver metastasis. Death 5 months after diagnosis. | |
| 2012 | Song et al | 74 | M | NA | Acute abdominal pain | Irregular cauliflower-like mucosal lesion | 3.0 cm-sized tumor | IPMN & ductal adenocarcinoma | N/A | CK7, MUC5AC and MUC6 | Type 2 | N/A | NA |
| 2015 | Yamaoka et al | 69 | F | NA | None | Irregularly shaped depression with red color | 2.8 × 2 cm | Ductal adenocarcinoma | Present | CK7, MUC5AC and MUC1 | Type 2 | N/A | Peritoneal carcinomatosis (9 months) |
| 2017 | Neal et al | 75 | F | 100 cm | Acute abdominal pain, nausea | NA | 4.7 × 4.2 × 3.7 cm | Ductal adenocarcinoma | NA | NA | not specified | yes | NA |
| 2017 | Yogi et al | 82 | M | 40 cm | Emesis | NA | NA | Ductal adenocarcinoma | NA | NA | Type 1 | NA | NA |
| 2019 | Lara et al | 81 | F | NA | Obstructive GI symptoms | NA | NA | Ductal adenocarcinoma | NA | NA | Type 2 | NA | Uneventful (12 months) |
| Year . | Author . | Age . | Sex . | Distance from LT . | Symptoms . | Appearance . | Size . | Histology . | LNs mets . | Immunostains . | Heinrich . | Chemotherapy . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1988 | Persson et al | 85 | F | 100 cm | NA | NA | 1.5 cm | NA | N/A | NA | Type 1 | N/A | NA |
| 1990 | Fujiki et | 54 | M | 50 cm | NA | NA | 3 × 2 cm | NA | NA | NA | Type 1 | NA | NA |
| 1993 | Satoh et al | 64 | M | 110 cm | Abdominal pain | NA | 2 × 1.5 cm | Ductal adenocarcinoma | NA | NA | Type 1 | NA | NA |
| 1999 | Makhlouf et al | 71 | M | unknown | None | Central ulceration | 1.5 cm ulcer, 3.5 cm tumor | Acinar adenocarcinoma | None | CK7, alpha1-antitrypsin, alpha1-antichymotrypson, BER-EP4 and CEA | Type 1 | N/A | Liver metastasis (1 year) |
| 1999 | Makhlouf et al | 61 | M | 8 cm | Intestinal obstruction | Cauliflower-like mucosal lesion | 1.5 cm | Ductal adenocarcinoma | Present | NA | Type 1 | NA | NA |
| 1999 | Arao et al | 63 | M | 20 cm | Epigastric discomfort, vomiting | NA | 4 × 2 cm | Ductal adenocarcinoma | N/A | NA | Type 2 | N/A | NA |
| 2007 | Sato et al | 78 | F | 50 cm | Abdominal pain, nausea, vomiting | Yellowish-brown protruding tumor | 3 × 2 cm | Ductal adenocarcinoma | NA | NA | Type 2 | NA | Uneventful |
| 2008 | Fujita et al | 64 | F | NA | Distension, epigastric pain | Ulcerated tumor | 2 cm | NA | Present | NA | yes | Peritoneal carcinomatsis. Death 5 months. after diagnosis. | |
| 2010 | Fujiwara et al | 76 | F | NA | Post pandrial vomiting | 2 × 1.5 cm | Ductal adenocarcinoma | Present | NA | Type 3 | N/A | Liver metastasis. Death 5 months after diagnosis. | |
| 2012 | Song et al | 74 | M | NA | Acute abdominal pain | Irregular cauliflower-like mucosal lesion | 3.0 cm-sized tumor | IPMN & ductal adenocarcinoma | N/A | CK7, MUC5AC and MUC6 | Type 2 | N/A | NA |
| 2015 | Yamaoka et al | 69 | F | NA | None | Irregularly shaped depression with red color | 2.8 × 2 cm | Ductal adenocarcinoma | Present | CK7, MUC5AC and MUC1 | Type 2 | N/A | Peritoneal carcinomatosis (9 months) |
| 2017 | Neal et al | 75 | F | 100 cm | Acute abdominal pain, nausea | NA | 4.7 × 4.2 × 3.7 cm | Ductal adenocarcinoma | NA | NA | not specified | yes | NA |
| 2017 | Yogi et al | 82 | M | 40 cm | Emesis | NA | NA | Ductal adenocarcinoma | NA | NA | Type 1 | NA | NA |
| 2019 | Lara et al | 81 | F | NA | Obstructive GI symptoms | NA | NA | Ductal adenocarcinoma | NA | NA | Type 2 | NA | Uneventful (12 months) |
Reported cases of adenocarcinoma arising in heterotopic pancreatic tissue in the jejunum.
| Year . | Author . | Age . | Sex . | Distance from LT . | Symptoms . | Appearance . | Size . | Histology . | LNs mets . | Immunostains . | Heinrich . | Chemotherapy . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1988 | Persson et al | 85 | F | 100 cm | NA | NA | 1.5 cm | NA | N/A | NA | Type 1 | N/A | NA |
| 1990 | Fujiki et | 54 | M | 50 cm | NA | NA | 3 × 2 cm | NA | NA | NA | Type 1 | NA | NA |
| 1993 | Satoh et al | 64 | M | 110 cm | Abdominal pain | NA | 2 × 1.5 cm | Ductal adenocarcinoma | NA | NA | Type 1 | NA | NA |
| 1999 | Makhlouf et al | 71 | M | unknown | None | Central ulceration | 1.5 cm ulcer, 3.5 cm tumor | Acinar adenocarcinoma | None | CK7, alpha1-antitrypsin, alpha1-antichymotrypson, BER-EP4 and CEA | Type 1 | N/A | Liver metastasis (1 year) |
| 1999 | Makhlouf et al | 61 | M | 8 cm | Intestinal obstruction | Cauliflower-like mucosal lesion | 1.5 cm | Ductal adenocarcinoma | Present | NA | Type 1 | NA | NA |
| 1999 | Arao et al | 63 | M | 20 cm | Epigastric discomfort, vomiting | NA | 4 × 2 cm | Ductal adenocarcinoma | N/A | NA | Type 2 | N/A | NA |
| 2007 | Sato et al | 78 | F | 50 cm | Abdominal pain, nausea, vomiting | Yellowish-brown protruding tumor | 3 × 2 cm | Ductal adenocarcinoma | NA | NA | Type 2 | NA | Uneventful |
| 2008 | Fujita et al | 64 | F | NA | Distension, epigastric pain | Ulcerated tumor | 2 cm | NA | Present | NA | yes | Peritoneal carcinomatsis. Death 5 months. after diagnosis. | |
| 2010 | Fujiwara et al | 76 | F | NA | Post pandrial vomiting | 2 × 1.5 cm | Ductal adenocarcinoma | Present | NA | Type 3 | N/A | Liver metastasis. Death 5 months after diagnosis. | |
| 2012 | Song et al | 74 | M | NA | Acute abdominal pain | Irregular cauliflower-like mucosal lesion | 3.0 cm-sized tumor | IPMN & ductal adenocarcinoma | N/A | CK7, MUC5AC and MUC6 | Type 2 | N/A | NA |
| 2015 | Yamaoka et al | 69 | F | NA | None | Irregularly shaped depression with red color | 2.8 × 2 cm | Ductal adenocarcinoma | Present | CK7, MUC5AC and MUC1 | Type 2 | N/A | Peritoneal carcinomatosis (9 months) |
| 2017 | Neal et al | 75 | F | 100 cm | Acute abdominal pain, nausea | NA | 4.7 × 4.2 × 3.7 cm | Ductal adenocarcinoma | NA | NA | not specified | yes | NA |
| 2017 | Yogi et al | 82 | M | 40 cm | Emesis | NA | NA | Ductal adenocarcinoma | NA | NA | Type 1 | NA | NA |
| 2019 | Lara et al | 81 | F | NA | Obstructive GI symptoms | NA | NA | Ductal adenocarcinoma | NA | NA | Type 2 | NA | Uneventful (12 months) |
| Year . | Author . | Age . | Sex . | Distance from LT . | Symptoms . | Appearance . | Size . | Histology . | LNs mets . | Immunostains . | Heinrich . | Chemotherapy . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1988 | Persson et al | 85 | F | 100 cm | NA | NA | 1.5 cm | NA | N/A | NA | Type 1 | N/A | NA |
| 1990 | Fujiki et | 54 | M | 50 cm | NA | NA | 3 × 2 cm | NA | NA | NA | Type 1 | NA | NA |
| 1993 | Satoh et al | 64 | M | 110 cm | Abdominal pain | NA | 2 × 1.5 cm | Ductal adenocarcinoma | NA | NA | Type 1 | NA | NA |
| 1999 | Makhlouf et al | 71 | M | unknown | None | Central ulceration | 1.5 cm ulcer, 3.5 cm tumor | Acinar adenocarcinoma | None | CK7, alpha1-antitrypsin, alpha1-antichymotrypson, BER-EP4 and CEA | Type 1 | N/A | Liver metastasis (1 year) |
| 1999 | Makhlouf et al | 61 | M | 8 cm | Intestinal obstruction | Cauliflower-like mucosal lesion | 1.5 cm | Ductal adenocarcinoma | Present | NA | Type 1 | NA | NA |
| 1999 | Arao et al | 63 | M | 20 cm | Epigastric discomfort, vomiting | NA | 4 × 2 cm | Ductal adenocarcinoma | N/A | NA | Type 2 | N/A | NA |
| 2007 | Sato et al | 78 | F | 50 cm | Abdominal pain, nausea, vomiting | Yellowish-brown protruding tumor | 3 × 2 cm | Ductal adenocarcinoma | NA | NA | Type 2 | NA | Uneventful |
| 2008 | Fujita et al | 64 | F | NA | Distension, epigastric pain | Ulcerated tumor | 2 cm | NA | Present | NA | yes | Peritoneal carcinomatsis. Death 5 months. after diagnosis. | |
| 2010 | Fujiwara et al | 76 | F | NA | Post pandrial vomiting | 2 × 1.5 cm | Ductal adenocarcinoma | Present | NA | Type 3 | N/A | Liver metastasis. Death 5 months after diagnosis. | |
| 2012 | Song et al | 74 | M | NA | Acute abdominal pain | Irregular cauliflower-like mucosal lesion | 3.0 cm-sized tumor | IPMN & ductal adenocarcinoma | N/A | CK7, MUC5AC and MUC6 | Type 2 | N/A | NA |
| 2015 | Yamaoka et al | 69 | F | NA | None | Irregularly shaped depression with red color | 2.8 × 2 cm | Ductal adenocarcinoma | Present | CK7, MUC5AC and MUC1 | Type 2 | N/A | Peritoneal carcinomatosis (9 months) |
| 2017 | Neal et al | 75 | F | 100 cm | Acute abdominal pain, nausea | NA | 4.7 × 4.2 × 3.7 cm | Ductal adenocarcinoma | NA | NA | not specified | yes | NA |
| 2017 | Yogi et al | 82 | M | 40 cm | Emesis | NA | NA | Ductal adenocarcinoma | NA | NA | Type 1 | NA | NA |
| 2019 | Lara et al | 81 | F | NA | Obstructive GI symptoms | NA | NA | Ductal adenocarcinoma | NA | NA | Type 2 | NA | Uneventful (12 months) |
Less common sites include the esophagus, ileum, Meckel diverticulum and biliary tree. Rare cases of HP tissue have also been found in sites as far as the mediastinum, lungs, spleen, fallopian tubes, umbilicus and omentum [1].
Two major theories have been proposed to explain the aberrant location of pancreatic tissue known as misplacement and metaplasia theories. The misplacement theory is most accepted one and claims that during the rotation of the dorsal and ventral buds in embryological development, some pancreatic tissue migrates from the main body of pancreas and deposits at different locations. This explains the occurrence of heterotopic pancreatic tissue in various parts of the gastrointestinal tract. [2–4]. Ectopic pancreatic tissue occurring in remote sites like neck and pelvis may have other pathogenesis and metaplasia or origin from teratoma may be more likely. [5]
There have been four different types of heterotopia described in the literature. Type I consists of typical pancreatic tissue with acini, ducts, and islet cells resembling normal pancreas. Type II includes pancreatic ducts and acini only. Type III is ducts with few acini or dilated ducts only. This type is also known as canalicular variety. Last, Type IV is endocrine pancreas conformed of islet cells only [6]. Majority of HP cases are asymptomatic. Therefore, it is difficult to ascertain its true incidence. According to autopsy studies, the incidence varies between 0.5 and 13.7%. [1]. Malignant transformation arising in heterotopic pancreas is extremely rare and ranges from 0.7% to 1.8% among all heterotopic pancreas cases [7].
As seen in this patient, it is usually a silent anomaly that can either be associated with inflammation, bleeding, obstruction, or malignant transformation. The patient then presents with the corresponding clinical picture. In regards to the diagnosis of malignant transformation, the histological study should fulfill three criteria: (1) the tumor must be located within or very close to the ectopic pancreatic tissue, (2) transition between benign pancreatic structures and carcinoma must be identified and (3) the non-neoplastic pancreatic tissue must comprise fully developed acini and ducts [8].
CONCLUSION
Heterotopic pancreatic tissue can be found in different locations along the gastrointestinal tract with a significant variability in location and presentation making its diagnosis very difficult. Although, this is a rare finding, it should be considered in the differential of pathological process associated to obstruction, inflammation, bleeding and or malignant transformation and presentation making its diagnosis very difficult.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.



