-
PDF
- Split View
-
Views
-
Cite
Cite
Yuma Hane, Takahiro Tsuchikawa, Satoshi Takeuchi, Kimitaka Tanaka, Yoshitsugu Nakanishi, Toshimichi Asano, Takehiro Noji, Yo Kurashima, Yuma Ebihara, Soichi Murakami, Toru Nakamura, Keisuke Okamura, Toshiaki Shichinohe, Satoshi Hirano, Long-term survival after repetitive lymphadenectomy for nodal recurrence of pancreatic neuroendocrine neoplasms: a report of two cases, Journal of Surgical Case Reports, Volume 2021, Issue 10, October 2021, rjab446, https://doi.org/10.1093/jscr/rjab446
Close - Share Icon Share
Abstract
Pancreatic neuroendocrine neoplasms (PNENs) are rare, but their incidence has increased in recent years. Curative surgery is recommended in several global guidelines for resectable PNENs. Lymph node recurrence after R0 resection for PNENs is infrequent, and global guidelines recommend surgical resection for recurrence, if resectable. However, data on the prognosis after surgical resection for nodal recurrence of PNENs are limited. We herein report two cases in which long-term survival was achieved after repetitive lymphadenectomy for nodal recurrence of PNENs. In both cases, the pathological findings for primary PNEN showed well-differentiated neuroendocrine neoplasms and R0 resection was successfully performed. The Ki-67 index increased with each resection in both cases. Both patients showed long-term survival (10 and 14 years, respectively). Repetitive lymphadenectomy for nodal recurrence of PNENs may improve patient prognosis.
INTRODUCTION
Pancreatic neuroendocrine neoplasms (PNENs) are rare tumors with a reported incidence of 0.48 in 100 000 persons, but the number of cases has been increasing recently [1]. PNENs have a better prognosis compared with pancreatic cancer, 5-year survival rate of PNENs is reported 54% by SEER data base. However, PNENs with distant metastases have a poor prognosis, with a reported 5-year survival rate of 25% by SEER data base. In 2017, the World Health Organization classified PNENs into well-differentiated and poorly differentiated neuroendocrine neoplasms (NENs) from a morphological point of view, with the former being further subdivided into neuroendocrine tumors G1, G2 and G3 based on the status of cell proliferation [2]. Resectable PNENs have been reported to have a good prognosis [3–5]. Therefore, curative surgical resection is recommended by several guidelines for well-differentiated NENs, with regional lymphadenectomy in cases in which the tumor size is >2 cm, according to the therapeutic strategy for pancreatic ductal adenocarcinoma.
Recurrence after R0 resection for PNENs is uncommon, with a reported incidence of 9.7% [6]. The National Comprehensive Cancer Network and Japanese Neuroendocrine Tumors Society guidelines recommend curative surgical resection for recurrence, if resectable [7]. However, data on the prognosis of PNEN recurrence after surgical resection are limited. Here, we focused on nodal recurrences and reported two cases in which long-term survival was achieved after repetitive lymphadenectomy for nodal recurrence of PNENs.
CASE SERIES
Case 1
A 71-year-old woman underwent distal pancreatectomy with prophylactic local lymph node resection, left adrenal resection and partial gastric resection for PNEN. The pathological findings of the resected specimen were as follows: well-differentiated NEN, tumor size of 36 mm, negative resection margins, positive vascular invasion, no lymph node metastases and a Ki-67 index of 4%. Four years after the pancreatectomy, abdominal computed tomography (CT) revealed a regional lymph node recurrence located dorsal to the left renal vein (Fig. 1A); therefore, regional lymphadenectomy was performed. The pathological finding was metachronous nodal recurrence of PNEN (Ki-67 index: unknown). Five years after the first surgery, regional lymph node recurrence occurred, with invasion of the left kidney (Fig. 1B); therefore, left nephrectomy with lymphadenectomy was performed. The pathological finding was metachronous nodal recurrence of PNEN (Ki-67 index: 20%) with renal invasion. Five years after the pancreatectomy, follow-up CT and gadoxetic acid–enhanced magnetic resonance imaging (MRI) revealed local recurrence and multiple liver metastases (Fig. 1C and D). Daily oral sunitinib (37.5 mg) was initiated 5 years and 5 months after pancreatectomy; however, the patient developed fever and joint pain 2 weeks after initiating sunitinib. Therefore, sunitinib was switched to daily oral everolimus (10 mg). She maintained stable disease for 1 year and 3 months after the initiation of everolimus; however, follow-up CT revealed enlargement of multiple liver metastases. Therefore, everolimus was switched to weekly streptozocin (800 mg/m2). She maintained stable disease for 1 year and 6 months after the initiation of streptozocin. Streptozocin was switched to sunitinib with lanreotide because of the enlargement of multiple liver metastases. Sunitinib with lanreotide was administered for 1 year and was switched to amrubicin (30 mg/m2) 3 days per week due to the enlargement of multiple liver metastases. The patient died of liver metastases 10 years after pancreatectomy.
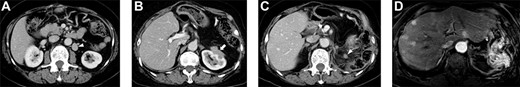
Selected abdominal computed tomography and gadoxetic acid–enhanced magnetic resonance images: Case 1. (A) Recurrence for the first time: para-aortic mass measuring 28 mm located dorsal to the left renal vein (arrowhead). (B) Recurrence for the second time: mass measuring 40 mm invading the left kidney (arrow). (C, D) Recurrence for the third time: mass measuring 12 mm at the same site as that of the second recurrence (c; arrowhead) and multiple liver metastases in the bilateral lobe (d).
Case 2
An 80-year-old woman underwent distal pancreatectomy with lymphadenectomy for PNEN. The pathological findings were as follows: well-differentiated NEN, tumor size of 80 mm, negative resection margins, negative lymphovascular invasion, no lymph node metastases and a Ki-67 index <1%. Five years after the pancreatectomy, nodal metastasis was noted near the abdominal aorta on positron emission tomography and CT (Fig. 2A). A para-aortic lymphadenectomy was performed. The pathological finding was metachronous nodal recurrence of PNEN and a Ki-67 index of 10%. Nine years after the pancreatectomy, nodal metastasis recurred near the abdominal aorta (Fig. 2B). We performed a second para-aortic lymphadenectomy, and the pathological finding was metachronous nodal recurrence of PNEN, with a Ki-67 index of 15%. Eleven years after the pancreatectomy, a third metachronous nodal recurrence appeared near the abdominal aorta (Fig. 2C). A third para-aortic lymphadenectomy was not performed because of severe aortic stenosis. Since somatostatin receptor scintigraphy revealed positive SSTR-2, 120 mg of lanreotide was administered. She complained of diarrhea as a side effect. The patient maintained stable disease for 14 years after pancreatectomy.
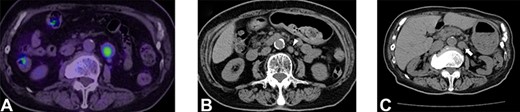
Selected abdominal computed tomography and positron emission tomography–computed tomography images: Case 2. (A) Recurrence for the first time: para-aortic mass measuring 24 mm with accumulation on positron emission tomography (SUVmax: 5.1). (B) Recurrence for the second time: para-aortic mass measuring 15 mm (arrowhead). (C) Recurrence for the third time: para-aortic mass measuring 24 mm (arrow).
DISCUSSION
In this report, we describe the cases of two patients who underwent repetitive lymphadenectomy for nodal recurrence after curative surgical resection of PNENs and achieved long-term survival.
In both cases, para-aortic lymph node recurrence occurred despite the absence of lymph node metastases in the pathological findings of the first surgery, which was caused by skip metastasis. Li et al. [8] reported that the mechanisms of skip metastasis are the following: occult metastasis or micrometastasis to N1 nodes may have been missed during dissection or routine histopathological examination; aberrant lymphatic drainage patterns may have been present in patients with gastric cancer through which metastasis bypasses the lymphatic vessels; lymphatic flow to the N1 nodes may have been blocked by cancer tissue and free cancer cells may diffuse through regional nodes to distant nodes because the microenvironment in N1 nodes is unsuitable for the development of metastasis [8]. Recurrence after R0 resection for PNEN is rare, with a reported incidence of 9.7% (43/462) and a reported nodal recurrence rate after R0 resection of 1.3% [9]. In a previous study, patients who underwent resection for metachronous local recurrence and liver metastases after R0/1 resection of PNENs had a better prognosis than those who did not [3]; therefore, global guidelines allow curative surgical resection of recurrence sites, including nodal recurrence, if it appears to be resectable on several imaging modalities [7]. However, due to its slow growing nature and recent advance in multimodal therapeutic modalities, it is difficult to clarify whether the surgical intervention alone separately contributed to the patients prognosis [10]. Moreover, there is a lack of data on the location of lymph node recurrence and prognosis with lymph node recurrence after surgical resection of PNENs. In our cases, repetitive lymphadenectomy for nodal recurrences resulted in long-term survival for 10 and 14 years.
In our department, all candidates for surgical resection are principally observed for at least 3 months after systemic treatment, if indicated. If a new metastatic lesion was found during this window period, surgical resection was not performed, suggesting systemic disease. To detect new metastatic lesions, cross-sectional imaging studies, such as CT, ultrasound, MRI and stereotactic radiosurgery (SRS) are used. In particular, MRI is useful for the detection of liver metastases, whereas SRS is useful for detecting lymph node and bone metastases [9, 11].
In the present cases, the Ki-67 index was elevated for each metastasis. Recently, some studies have reported that the Ki-67 index may increase throughout the disease course in NENs due to genetic intramural heterogeneity or therapy resistance [12, 13]. Moreover, the duration to the next recurrence was shortened over the course of the disease, with an increase in the Ki-67 index. This could be interpreted as a change in tumor pathology during disease progression. Re-biopsy, functional imaging modalities and an appropriate window period are required to make a decision on the operative indication for lymph node recurrence in rapidly progressive cases.
In conclusion, repetitive lymphadenectomy for resectable nodal recurrence of PNENs with multidisciplinary treatment may contribute to improved prognosis.
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.jp) for English language editing.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patients for publication of this case report and the accompanying images.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests relevant to this article.
FUNDING
None.
References
Manisha HS, Whitney SG, Benson III AB, Emily B, Lawrence SB, Pamela B, et al. Neuroendocrine and adrenal tumors, version 2. 2021.



