-
PDF
- Split View
-
Views
-
Cite
Cite
Jared McNeill, Hong Chew, David Andresen, David Muller, Emily Granger, Louis W Wang, Coronary artery embolism and culture-negative endocarditis post Bentall’s procedure, Journal of Surgical Case Reports, Volume 2021, Issue 10, October 2021, rjab438, https://doi.org/10.1093/jscr/rjab438
Close - Share Icon Share
Abstract
Infective endocarditis is an important cause of morbidity and mortality, which classically presents with fevers and nonspecific symptoms. Afebrile infective endocarditis with negative blood cultures makes diagnosis more challenging and delays in treatment can occur increasing the likelihood of complications. The presence of prosthetic heart valves places patients at an increased risk of infective endocarditis and the case described below highlights the importance of considering this diagnosis even if classic clinical features such as fever and raised inflammatory markers are not present, as well as discussing an unusual complication of infective endocarditis; coronary artery embolism leading to myocardial infarction.
INTRODUCTION
Infective endocarditis is a significant cardiac disease with high morbidity and mortality [1]. Patients commonly present with fever and nonspecific symptoms, such as fatigue, myalgias, headache or dyspnoea. On examination, patients may have a new cardiac murmur and stigmata of infective endocarditis such as splinter hemorrhages, Osler nodes or Janeway lesions. The presence of prosthetic heart valves places patients at an increased risk of infective endocarditis [2] and the case described below highlights the importance of considering this diagnosis even if classic clinical features such as fever are not present.
CASE REPORT
A 65-year-old Caucasian male presented to a routine follow up 18 months after a Bentall’s procedure with bioprosthetic aortic valve replacement and hemiarch repair for severe asymptomatic aortic valve regurgitation and aortic root dilatation. He reported a 1-month history of fatigue and malaise, as well as occasional short, self-limiting episodes of tongue paresthesia. No associated fevers were reported. His recovery from the original surgery had been complicated by a cardiac tamponade on postoperative Day 4, which required emergency surgery and repair of a tear at the aortic annulus. Other medical history included hypertension and reflux oesophagitis. Regular medications included aspirin, atenolol and amlodipine.
On examination, he was afebrile and with no peripheral stigmata of endocarditis. However, cardiac auscultation revealed an additional heart sound. Suspecting prosthetic valve vegetation or thrombosis, an urgent transthoracic echocardiogram was performed. This demonstrated a mobile mass associated with the prosthetic aortic valve.
The patient was immediately admitted for investigations and consideration of urgent cardiothoracic surgery. Blood tests showed a white cell count (WCC) of 7.6 × 109/l and c-reactive protein (CRP) of 1.1 mg/l. Serial blood cultures were sent, all of which showed nil growth. Transesophageal echocardiography confirmed a 1.5 cm × 0.4 cm vegetation attached to the ventricular aspect of the aortic valve bioprosthesis with trivial aortic regurgitation. Thickening of the aortic root was also evident, suggesting infective involvement of the aortic root. Chest computed tomography demonstrated a contained collection around the Bentall’s graft. Brain computed tomography and magnetic resonance imaging demonstrated no acute intracranial hemorrhage, nor the presence of cerebral embolism or mycotic aneurysm.
On Day 1 of his admission the patient developed sudden onset chest pain radiating to the back, associated with electrocardiogram features of an anterior ST elevation myocardial infarction (Fig. 1). He was taken for an urgent coronary angiogram, which demonstrated proximal occlusion of the left anterior descending artery (LAD) secondary to coronary artery embolism (Fig. 2). A guidewire was successfully passed into the LAD, and an aspiration-thrombectomy catheter was employed to successfully re-canalize the occluded artery.
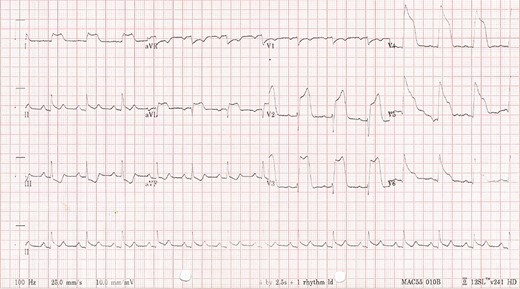
Patient’s electrocardiogram day one of admission demonstrating anterior ST elevation myocardial infarction with reciprocal ST-T wave changes in the inferior leads.
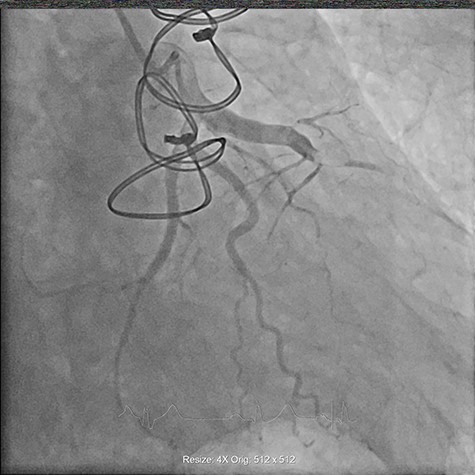
Coronary angiogram demonstrating proximally occluded LAD artery.
Due to concerns of a recurrent systemic embolism, the patient then underwent redo aortic valve replacement and hemiarch replacement. The Bentall’s graft was found to be thickened and surrounded by a layer of pus. The presence of inflammation was confirmed on histological examination of the excised graft. A typical endocarditic lesion was attached to the non-coronary cusp of the bioprosthetic valve in the region of a previous annular tear and repair (Fig. 3). The infected material was removed, and the original graft replaced with a homograft. Given the purulent contamination found, the mediastinum was packed at the end of the operation, with the intention of performing a staged closure. This was successfully completed 2 days later, with laparoscopic mobilization of the omentum to surround the new homograft to reduce the risk of future infection.
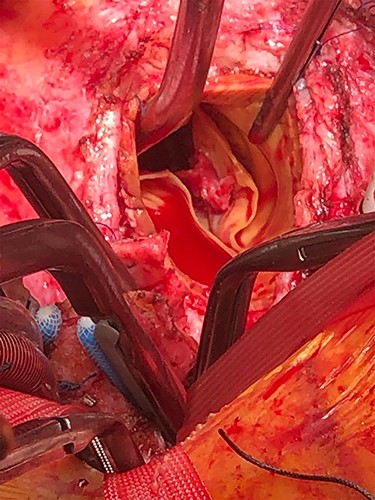
Endocarditic lesion on the ventricular surface of the non-coronary cusp of the bioprosthetic valve during revision of Bentall’s procedure.
Intra-operative tissue cultures grew Staphylococcus saccharolyticus, Staphylococcus cohnii and Cutibacterium acnes. Following successful surgery, the patient received intravenous ceftriaxone and vancomycin for 6 weeks, followed by 6 months of oral amoxicillin. Eighteen months following surgery, the patient remains well. Progress echocardiography demonstrated a normal functioning prosthetic aortic valve, normal left ventricular size with mild residual hypokinesis in the left ventricular anterior wall (Ejection fraction: 50%).
DISCUSSION
This case illustrates an important, life-threatening example of systemic embolism related to prosthetic valve vegetation. The lack of fever, systemic features of infection (including normal WCC and CRP) and negative blood cultures in an immunocompetent patient with such extensive local infection of his aortic root is highly unusual. Fever is present in ~90% of cases of infective endocarditis, and only 10% of patients have no growth on blood cultures [3]. Afebrile presentations with negative cultures can occur when endocarditis is caused by fastidious organisms or skin commensals [4], as was found in this case.
Similar cases are rarely reported in the literature. A literature review using Ovid MEDLINE identified only 11 other published reports of afebrile culture-negative endocarditis between 1960 and 2021 [5–15]. Of the reported cases, our case was the only identified in the literature to be caused by skin commensals. Bartonella species and Q fever (Coxiella burnetii) were most commonly identified as causative organisms, being present in 6 out of 11 reports [7–10, 13, 15]. Other causes include Enterococcus5, Enterobacter6, Brucella14 and Tropheryma whippelii11. Coronary artery embolism was not identified as sequelae in any other reported case of afebrile culture-negative endocarditis.
Matsukuma et al. discuss a similar case of afebrile infective endocarditis with negative cultures and normal inflammatory markers, involving a prosthetic mitral valve. Enterococcus Faecalis was identified from vegetation cultures following surgery. This patient similarly experienced embolic events (left hemiparesis from multiple embolic infarcts) prior to surgical management of the infected prosthetic valve. Giladi et al. described a case of afebrile culture-negative endocarditis caused by Enterobacter in a patient with rheumatoid nodules on their mitral valve, who presented with embolic events (right sided weakness and speech difficulties). However, in this case inflammatory markers were raised (WCC 19.06 × 109/l). These cases highlight that embolic events often occur before surgical management is arranged. Early surgical intervention should be advocated for in cases of afebrile culture-negative endocarditis once valve vegetations have been identified.
Clinical vigilance and surveillance for symptoms remains an important part of the postoperative management of patients with a history of cardiac valvular surgery. The presence of any symptoms or signs suggestive of valve dysfunction or systemic embolic phenomenon in a patient with a prosthetic valve or previous aortic root surgery warrants urgent investigations to exclude the presence of infective endocarditis or sterile thrombotic vegetation.
AUTHORS' CONTRIBUTIONS
JM, HC, DA, DM, EG and LWW contributed to the care of the patient. All authors wrote the report.
CONSENT
Written consent to publish was obtained.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



