-
PDF
- Split View
-
Views
-
Cite
Cite
Blessie Nelson, Dylan Warfield, Camilo Velasquez Mejia, John Patrick Walker, Rare presentation of growing teratoma syndrome in patient with remote history of testicular cancer resection, Journal of Surgical Case Reports, Volume 2021, Issue 1, January 2021, rjaa600, https://doi.org/10.1093/jscr/rjaa600
Close - Share Icon Share
Abstract
Growing teratoma syndrome (GTS) is documented in literature to be a rare complication of non-seminomatous germ cell tumors that arises following chemotherapeutic treatment. Though represented through multiple case reports, the condition is rare that it evades observation and diagnosis, leading to complications secondary to metastasis and unchecked growth. GTS is identified via incidental finding on imaging (e.g. CT) or due to complications involving mass obstruction. Due to the potential severity of undiagnosed malignancy, it is important to effectively diagnose GTS in those presenting with non-specific symptoms and a history of testicular/ovarian cancer. It is also necessary to develop a method on how to monitor those considered to be at increased risk for developing such a condition. Here, we present a case of a middle-aged male who presented with complaints of a left lower quadrant abdominal mass and incidental finding of right retroperitoneal lesion, consistent with GTS.
INTRODUCTION
Growing teratoma syndrome (GTS) is a rare complication of non-seminomatous germ cell tumors (NSGCTs) that arises following chemotherapy, comprised entirely of mature teratomatous cells. GTS typically presents within 2 years of initial treatment after recurrence of mass growth and/or metastasis. Here, we present a case of a GTS presenting as a left lower quadrant mass with ulceration, 19 years after treatment of a primary testicular cancer.
CASE REPORT
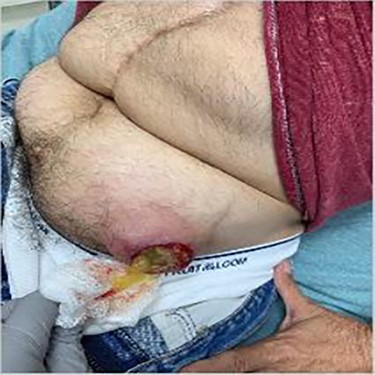
A 56-year-old male with a history of left testicular cancer treated with chemotherapy (Bleomycin, Etoposide, Cisplatin; BEP), radiotherapy, retroperitoneal lymph node dissection, and partial left nephrectomy in 1992 presented to hospital with complaints of left lower abdominal mass for 3–5 years, associated with ulceration and active drainage. He was asymptomatic with β-hCG and alpha-fetoprotein (AFP) levels within reference ranges (<2.39 and 1.4, respectively). On examination, inspection of abdomen revealed well-healed midline scar with bilateral incisional hernias and a 10 × 8 cm mass in the left lower quadrant (Fig. 1) firm and fixed to the abdominal wall. An open lesion characterized by yellow, non-purulent drainage was noted in the left lower quadrant. CT imaging of abdomen and pelvis with contrast revealed a multiloculated large cystic mass in the left inguinal canal measuring 11 × 7 × 7.7 cm (Fig. 2) along with a similar soft tissue nodule in the right inguinal area. A right retroperitoneal soft tissue mass in the mid abdomen was also identified, measuring 3.3 × 4.2 × 5.7 cm (Fig. 3). Changes of prior left nephrectomy and orchiectomy consistent with treatment of previous left-sided testicular cancer were also noted.
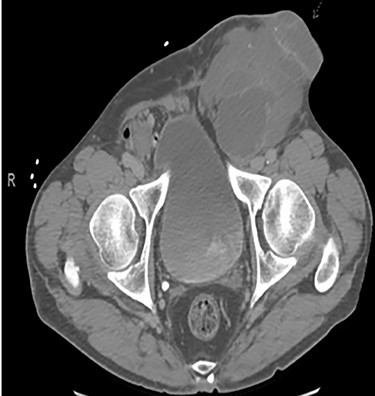
CT imaging abdomen and pelvis with contrast revealed a multiloculated large cystic mass in the left inguinal canal measuring 11 × 7 × 7.7 cm.
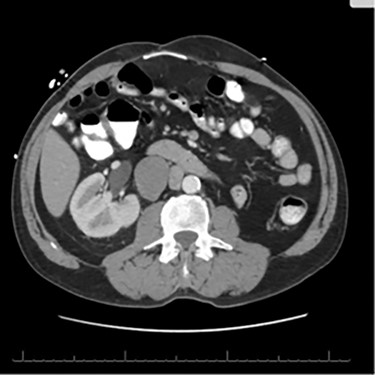
CT scan axial cut evidencing a right retroperitoneal mass measuring 3.3 × 4.2 × 5.7 cm.
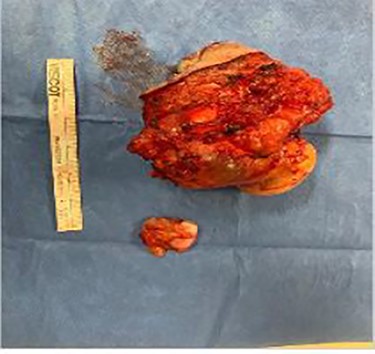
Biopsy of right retroperitoneal mass revealed squamous cell-lined cystic lesion followed by left lower quadrantabdominal mass excision and complex wound closure. An elliptical incision was made in the left groin incorporating the lesion, and subcutaneous flaps were created. Dissection was carried down to fascia where the mass extended into inguinal ring and the lower edge of the external oblique aponeurosis, extending into the abdomen. The mass was excised and noted to be multi-loculated and heterogeneous in consistency. The fascia and peritoneum that was adhered to the mass were then resected. A superficial inguinal lymph node was excised and sent to pathology as a separate specimen, and no other inguinal or femoral enlarged lymph nodes were found. Final pathology results revealed a mucinous cystic neoplasm with a negative lymph node for malignancy, consistent with potential spermatic cord mucinous cystadenoma (Fig. 4). The presence of retroperitoneal and contralateral inguinal masses, however, with history of teratoma raised suspicion of bilateral mucinous cystadenoma vs. residual/recurrent teratomatous component of testicular tumor. With this patient’s history and laboratory findings, diagnosis of recurrent teratoma was favored. The patient was asked to follow up with surgery two weeks after the procedure. At follow-up, patient denied any new onset of symptoms and was deemed to be experiencing appropriate postoperative recovery.
DISCUSSION
As originally defined by Logothetis et al., GTS is a rare condition characterized by an increasing mass, caused by teratomatous cells, following administration of chemotherapy. Diagnostic criteria include normalization of β-hCG and AFP, persistent enlargement of a mass following chemotherapy, and mature teratoma on pathological assessment, void of additional germ cell precursors, often originating from the testicles or ovaries [1]. The patient described in this report is a 56-year-old male who presented approximately 27 years after chemotherapeutic treatment for testicular cancer in 1992 with development of ulceration and drainage from abdominal mass that had been present for approximately 3–5 years. This is significant considering that development of GTS has been previously documented to typically arise within two-year period following completion of a chemotherapeutic regimen, specifically between 15 and 27 months [2]. Though there is reference in literature of cases being discovered as far as 19 years post-therapy, it is noted that this is a particularly rare occurrence in the setting of a pathology that, itself, is very underrepresented, with an approximate incidence of GTS ranging between 1.9 and 7.6 percent of confirmed NSGCTs [3]. Given our patient’s rare case, we seek to provide another uncommon presentation of GTS. Additionally, we perform a brief literature review for further insight into the characterization of GTS.
The exact pathophysiology/etiology of GTS is unclear, of which two of the more popular theories suggest the following: chemotherapy may induce malignant NSGCT cells to progress into mature teratomas [4] or it may lead to the death of all tumor cells excluding those associated with mature teratoma [5]. The primordial germ cell provides the origin for germ cell tumors (GCT), where successful genetic divergence (either via abnormal division, retention of embryonic features, or genomic instability) leads to the formation of malignant precursors defined as germ cell neoplasia in situ (GCNIS). Continuous alterations occur (e.g. chromosome 12p amplification and/or CCND2/KRAS/MDM2 mutations) to yield GCT, as described by Michelksi et al. [6]. GCNIS-derived teratomas are remarkable for the fact that they arise from these germ cell precursors, as proven by Jones et al., who discovered a correlation between metastatic mature teratomas and non-teratomatous GCT components by identifying several chromosomal locations (1p36, 9p21, 9q21, 13q22-q31, 18q21 and 18q22) that revealed identical genetic alterations between the mature teratoma and non-teratomatous samples alike [7]. This discovery provides a possible explanation for the theory suggesting that growing teratomas arise from teratomatous remnants of chemotherapy. Following the pattern of cell differentiation, it is reasonable to consider that mature teratomas may simultaneously reside with additional germ cell components in an individual diagnosed with NSGCT. Following eradication of germ cell components with chemotherapy, teratomatous components continue to differentiate, leading to recurrence of symptoms secondary to mass effect and/or metastasis.
CONCLUSION
GTS is a rare, evasive diagnosis that is difficult to qualify and develop standardized guidelines for. Though the consensus remains that complete excision of the mass is necessary, there is lack of appropriate guidelines for surveillance after treatment. This necessitates the development of standardized guidelines for GTS, regarding both post-treatment observation and pre-diagnostic screening for individuals with a history of NSGCTs.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
INFORMED CONSENT
Obtained from patient.



