-
PDF
- Split View
-
Views
-
Cite
Cite
Steven Liben Zhang, Hanjing Lee, Elijah Zhengyang Cai, Yan Lin Yap, Tseng Tsai Yeo, Thiam Chye Lim, Char Loo Tan, Jane Lim, Dermointegration in the exposed titanium cranioplasty: a possible protective phenomenon, Journal of Surgical Case Reports, Volume 2021, Issue 1, January 2021, rjaa551, https://doi.org/10.1093/jscr/rjaa551
Close - Share Icon Share
Abstract
Implant exposure is a known complication of titanium mesh cranioplasty and is usually managed by implant removal and/or exchange. We describe a case of exposed titanium mesh cranioplasty which was managed with implant exchange and bipedicled flap coverage, and showcase an interesting phenomenon of full-thickness skin present beneath the exposed mesh. This was confirmed on histopathology, which showed the presence of dermal appendages including pilosebaceous units and eccrine glands. We postulate that the mechanism behind this phenomenon involves islands of viable skin ‘dropping’ between holes in the mesh and coalescing beneath the exposed implant, as suggested by histopathology findings of nodular protrusions and varying degrees of epidermal hyperplasia. This protects the underlying dura from external infection. We propose for this phenomenon to be called dermointegration. Our findings suggest that similar cases, particularly patients who are not fit for general anaesthesia, may potentially be managed with a more conservative approach.
INTRODUCTION
Cranioplasty is commonly performed following craniectomy for various aetiologies including traumatic brain injury, stroke and malignancy. It is indicated to protect the brain and reduce aesthetic deformity associated with contour depression [1]. While a variety of materials are available [2], titanium is commonly used because it is hard, rigid, strong, light, resistant to infection, biologically inert, easily obtainable and a cheaper alternative to patient-specific implants [1, 2]. It is most frequently used in the form of a mesh plate.
Despite its benefits, the titanium cranioplasty implant is associated with complications, including thinning of the overlying soft tissue and implant exposure [3]. Implant exposure is particularly troublesome as it causes issues with hygiene and social embarrassment, poses a risk of secondary infection, compromises aesthetic outcome and usually necessitates implant removal and revision surgeries [4].
We report an interesting phenomenon noted in a case of exposed titanium mesh cranioplasty, where full-thickness skin was found beneath the exposed implant and confirmed on histopathology to include skin appendages like pilosebaceous units and eccrine glands. We postulate the mechanism behind this phenomenon and discuss the implications that this may have on the future management of similar cases.
CASE REPORT
A 54-year-old man presented with a three-month history of a right parietal scalp wound, measuring 5 × 2.5 cm, with exposed titanium mesh (Fig. 1A). He had a post-traumatic decompressive craniectomy and titanium mesh cranioplasty 18 years ago. A computed tomography scan of the brain did not reveal any underlying collection and showed the configuration of the titanium mesh which resulted in the exposure (Fig. 1B). He was counselled for and underwent wound exploration, implant removal and exchange, and bipedicled flap reconstruction.
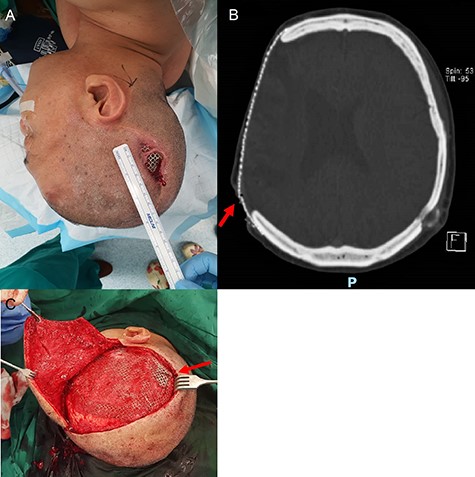
Parietal scalp wound with an exposed implant. A 54-year-old man presented with a right parietal scalp wound measuring 5 × 2.5 cm, with exposed titanium mesh cranioplasty implant (A). Computed tomography showed outward tenting of the implant beneath the area of exposure (B). This was correlated intraoperatively, due to venting cuts made from the initial surgery (C). This had likely caused pressure on the overlying skin and resulted in skin breakdown and implant exposure.
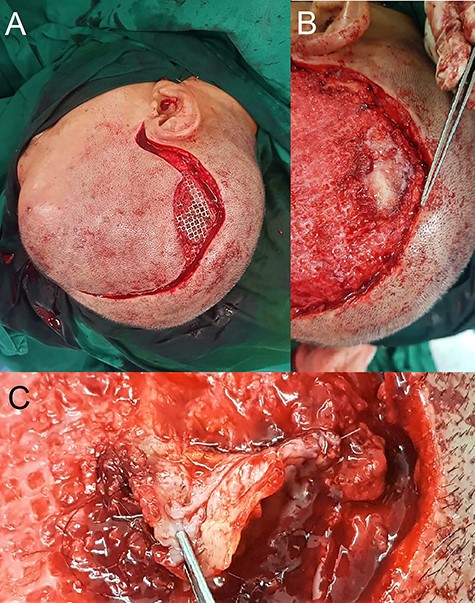
Intra-operative findings of hair-bearing skin beneath the exposed implant. The wound was debrided and incision extended along the previous scar (A). Upon removal of the implant, we found a layer of epithelium with hair follicles and surrounding granulation tissue beneath the area of the exposed implant (B, C); this was fully excised down to the dural covering layer and sent for histopathology.
Intraoperatively, venting cuts in the mesh were noted (Fig. 1C), which had likely caused pressure on the overlying skin, resulting in breakdown and implant exposure. Most interestingly, there was a layer of epithelium with hair follicles and surrounding granulation directly beneath the area of an exposed implant (Fig. 2). This was excised and sent for histopathology, which revealed dermal appendages including pilosebaceous units and eccrine glands, associated with prominent chronic inflammation (Fig. 3).
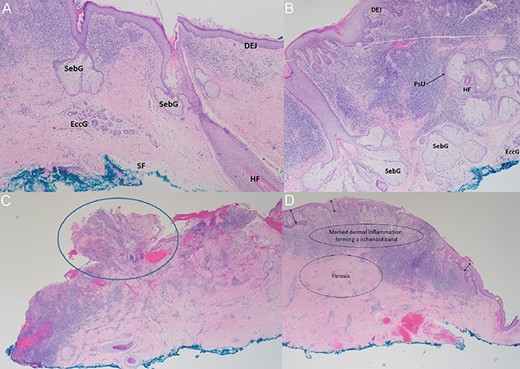
Histopathology findings. (A, B) Haematoxylin–Eosin staining (×40): micrographs showing skin with pilosebaceous units and prominent chronic inflammation in the superficial dermis with exocytosis. SebG = sebaceous gland; EccG = eccrine glands; DEJ = dermo-epidermal-junction; HF = hair follicle; PsU = pilosebaceous unit (black arrow). (C) Haematoxylin–Eosin staining (×20): Nodular protrusions of skin (blue circle), corresponding to gaps/holes within titanium mesh. (D) Haematoxylin–Eosin staining (×20): marked dermal inflammation with lichenoid bands and fibrosis, with varying degrees of epidermal hyperplasia (black double-arrows) lending support to our hypothesis of re-epithelialisation between skin islands. Epidermis is relatively thinner towards the edge of the specimen (yellow double-arrow), corresponding to the interface with the edge of the exposed implant.
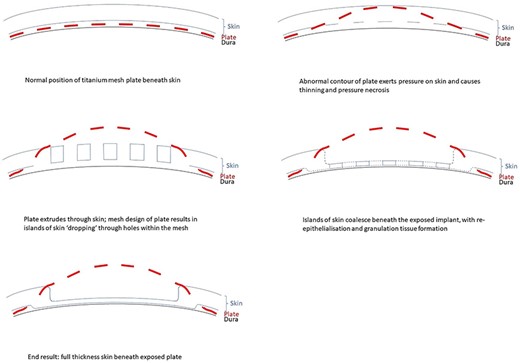
Proposed mechanism of dermointegration. In the normal patient, the titanium plate lies between the dura and overlying scalp skin (top left). Local factors such as abnormal plate contour exert pressure on the skin and causes thinning and pressure necrosis (top right). The plate extrudes through the skin, and the mesh plate design results in islands of the skin ‘dropping’ through holes within the mesh (middle left). Islands of skin coalesce beneath the exposed implant, with re-epithelialisation and granulation tissue formation (middle right). The eventual result is a layer of full-thickness skin overlying and protecting the dura beneath the exposed plate (bottom left).
DISCUSSION
Implant exposure is a known complication of titanium mesh cranioplasty, occurring in about 14% of patients [3, 4]. Standard management involves wound debridement, implant removal and/or exchange, with the aims of preventing secondary infection and maintaining an acceptable aesthetic outcome [4]. Resultant skin and soft tissue defects are often sizeable, and require reconstruction with locoregional and/or free flaps [5]. This adds to the duration of surgery, introduces potential donor site morbidity, and increases surgical and anaesthetic risks for patients who often have concomitant neurological risk factors, including seizures and cerebrovascular disease [6].
From our institutional experience, we have managed patients with exposed titanium implants who did not undergo surgery, due to significant comorbidities which increase anaesthetic risks. Surprisingly, these patients continue to survive for years without infection. Parallels can be drawn with similar situations such as osseointegrated dental implants and tissue expanders with external ports, where foreign bodies are exposed to the external environment in a stable state with minimal risk of infection [7, 8]. We hypothesise that the layer of full-thickness skin beneath the exposed implant, as shown in our patient and histopathology, could be protective. Intraoperatively, there is macroscopic evidence of granulation tissue with islands of hair-bearing skin directly beneath the exposed implant (Fig. 2). Histopathology confirmed microscopic features of full-thickness skin, with dermal appendages including pilosebaceous units and eccrine sweat glands, and even subcutaneous fat (Fig. 3). This growth of skin has likely integrated into the dural covering as would a skin graft and acts as a protective barrier against infection. A review of the literature did not reveal any published reports of such a phenomenon. We propose for this phenomenon to be called ‘dermointegration’.
While the exact mechanism behind this phenomenon remains unclear, we can understand the pathophysiology by reviewing a crucial step in the proliferative process of wound healing—epithelialisation, which is marked by proliferation and migration of keratinocytes [9]. Keratinocytes are regenerated from stem cells within the pilosebaceous units, eccrine sweat glands and outer root sheath of the hair follicle [10], which differentiate into keratinocytes and repopulate the stratum basale [9]. These keratinocytes migrate across the wound, proliferating at its edges until they meet in the middle [10]. This epithelial layer protects the wound from infection and desiccation [10].
We postulate that re-epithelialisation plays an essential role in the phenomenon of dermointegration. The initial skin breakdown occurs as a result of pressure from a focal area of the poorly contoured titanium mesh, more commonly seen in older plates where less attention might have been given to proper moulding. While the skin overlying the protruding metal undergoes necrosis, small islands of viable skin or keratinocytes may ‘drop’ through the holes of the titanium mesh (Fig. 4). These skin islands are visible on histological slides as nodular protrusions (Fig. 3C). Re-epithelialisation occurs as these keratinocytes coalesce beneath the exposed implant and over the underlying dura, repopulating and forming a continuous epithelial layer which acts as a protective barrier between the dura and the environment. This is in contrast to healing by contraction, which results in a scar without dermal appendages [10]. In addition, varying degrees of epidermal hyperplasia on histopathology (Fig. 3D) lends support to the hypothesis of re-epithelialisation between islands of viable skin.
In conclusion, this case provides evidence of full-thickness skin growth beneath an exposed titanium mesh cranioplasty implant, a phenomenon which we describe as ‘dermointegration’. This has a potential impact on the management of similar cases in the future, specifically in patients who are poor candidates for long surgery due to concomitant neurological risk factors, individual preference, or cost concerns. In such patients, one may consider the option of simply removing the portion of an implant that is exposed and leaving the underlying skin as functional soft tissue coverage (while accepting some degree of suboptimal aesthetic contour). Further studies are required to better understand the mechanism behind this phenomenon, and to investigate the long-term outcome of patients who are managed conservatively.



