-
PDF
- Split View
-
Views
-
Cite
Cite
Rashmi Seth, Genia Dubrovsky, Ronald W Busuttil, Robert B Cameron, Management of a large delayed esophageal perforation in a fresh liver transplant patient with endoscopic placement of a nasopleural drainage tube—a case report, Journal of Surgical Case Reports, Volume 2020, Issue 9, September 2020, rjaa385, https://doi.org/10.1093/jscr/rjaa385
Close - Share Icon Share
Abstract
Esophageal perforation in liver transplant recipients is a rare phenomenon. We herein report a case of an esophageal perforation due to Sengstaken–Blakemore tube in a liver-transplant recipient diagnosed 6 weeks post-transplant. A 2.5-cm mid-esophageal perforation communicating with large complex fluid collection in the pleural space was found. During endoscopy, 16Fr Salem Sump nasopleural tube (NP) was placed traversing through esophageal perforation into inferior aspect of the collection. Over the following 4 weeks, NP decompressed the cavity, allowed its closure and the tube was slowly retracted. By the end of 4 weeks, NP was removed with follow-up esophagogram showing no extravasation of contrast and a healed perforation. Hence, the esophageal perforation was successfully treated via this unique nonoperative approach without the need for major surgery. In instances of chronic leak with a stable patient, this nonoperative strategy should be considered even in immunocompromised patients.
INTRODUCTION
Esophageal perforation has traditionally been considered a life-threatening event with mortality rates of 10–40% [1]. In liver transplant recipients, it is a rare phenomenon. Among complications of portal hypertension, variceal bleeding is fairly common [2]. Frequently, Sengstaken–Blakemore (SB) tubes are used for emergency hemostasis, and although rare, it can cause esophageal rupture that can be fatal [3]. The usual treatment for esophageal perforation is to perform surgical drainage of the mediastinum and pleural space followed by primary repair of the defect with tissue coverage [4]. However, in some select cases, nonoperative management has been attempted [5]. We herein report a case of an esophageal perforation in a liver transplant recipient diagnosed 6 weeks post-transplant and treated successfully nonoperatively.
CASE SUMMARY
This is a 62-year-old female with chronic liver failure secondary to Hepatitis C who had undergone multiple transarterial chemoembolization in the past. She presented to emergency department in November 2019 with hematemesis and underwent upper endoscopy (esophagogastroduodenoscopy [OGD]), which showed nonbleeding esophageal and gastric varices with no stigmata of recent bleed. She was transferred to University of California Los Angeles for further management. Fourth day postadmission, she had massive hematemesis, became unresponsive and aggressively resuscitated. She was intubated and underwent SB tube insertion followed by an emergent embolization (coil/glue) of gastric varices and splenorenal shunt, followed by transjugular intrahepatic portosystemic shunt (TIPS). TIPS was further complicated by portal vein thrombus requiring balloon angioplasty and an additional stent within TIPS. She remained in the intensive care unit in critical condition intubated, on vasopressors and dialysis. Upon multidisciplinary discussion at selection committee, she was accepted as a candidate for liver transplant.
Five weeks postadmission, she underwent orthotopic liver transplant using a 55-year old brain dead donor. She had a model for end-stage liver disease score of 40 and blood type B. Native hepatectomy was challenging. She had severe portal hypertension and required systemic venovenous bypass and intraoperative dialysis. Her portal vein was sclerotic and thrombosed and required an endovenectomy. Cold ischemia time was 12 h. Anastomosis were standard: caval replacement, end-to-end portal vein, donor celiac trunk with recipient branch patch of gastroduodenal/hepatic artery and choledocho-choledocostomy. Over next 3 weeks, she made good recovery with functioning allograft, eating a regular diet and discharged 8 weeks postadmission. She received standard immunosuppression with steroids, tacrolimus and mycophenolate mofetil.
Three weeks postdischarge, she was re-admitted with fevers and shortness of breath and was found to be fluid overloaded. Esophagogram showed a large 2.5-cm defect in the mid-distal esophagus with extravasation of contrast material into right pleural cavity without communication to the bronchial tree (Fig. 1). An OGD further corroborated these findings. 16F Salem nasogastric tube was advanced into esophagus and placed into the bottom of the right pleural space.10F nasoduodenal feeding tube was also placed.
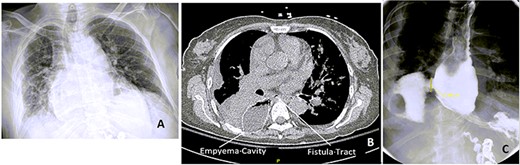
Chest x-ray (CXR), CT chest and esophagogram on the day of admission. (A) CXR showing right-sided pleural effusion and consolidation, (B) CT chest showing the empyema cavity and the fistula tract, (C) esophagogram showing a 2.5-cm mid-distal esophageal defect with contrast extravasting into the right pleural space.
The patient remained nil per os (NPO), started on antibiotics (piperacillin/tazobactum), antifungals (fluconazole) and enteric feeds. Figure 3 shows a schematic of the timing of esophagograms, nasopleural tube (NP) retraction and introduction of diet in relation to that.
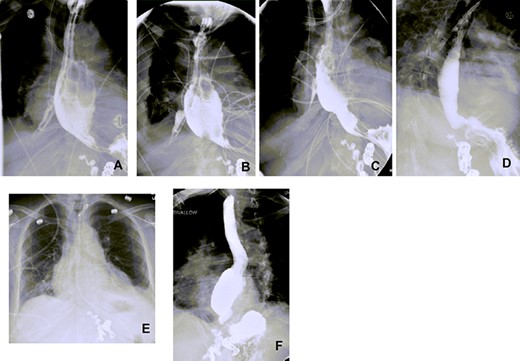
Esophagograms at various stages postadmission showing improvement in the size of the esophageal defect. (A and B) d13 and d19 Postadmission, respectively. NP tube in place ending into the right pleural space cavity. (C) d26 Postadmission with NP tube now retracted 5 cm, with decrease in the contrast extravasation seen. (D) d33 Postadmission with NP tube now removed showing no contrast extravasation seen with defect now healed. (E) CXR d41 postadmission showing improved right-sided pleural effusion and consolidation with no NP tube in place. (F) Outpatient follow-up esophagogram (d64 postadmission), (NP—nasopleural).
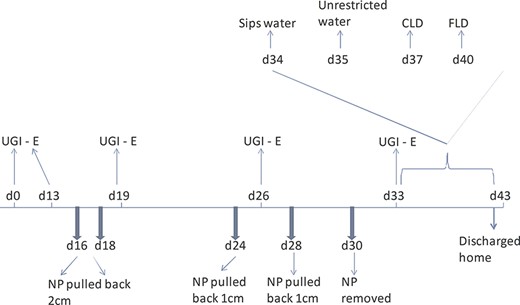
Schematic showing timing of esophagograms and retraction of NP tube along with introduction of diet. d0 is the day of admission/diagnosis (UGI-E—upper gastrointestinal series (esophagogram), NP nasopleural tube, CLD clear liquid diet, FLD full liquid diet).
DISCUSSION
Esophageal perforation in liver transplant patients is uncommon with paucity of data in the literature describing its management. Only a single report from 1990s by Dr Starzl describes the nature and treatment of major esophageal complications seen in 7 (0.6%) of 1154 adult liver transplant recipients at the University of Pittsburgh between Jan 1986 and March 1990 [6]. Three of the four patients with esophageal perforation died from 2 to 198 days after diagnosis in spite of operative treatment. Hence, timely diagnosis and prompt decision for appropriate treatment strategy is important.
Variceal bleeding is common with severe portal hypertension [2]. In such catastrophic bleeding, SB tubes can be used to temporarize the situation. SB tubes can be associated with several complications including tube displacement, esophageal perforation and mucosal necrosis [3]. With immediate diagnosis of perforation, immediate removal of tube, upper endoscopy and use of metallic covered stents have been successfully used where bleeding was controlled endoscopically [7]. In another report, endoscopic clipping to repair SB-associated 2.5-cm perforation has been described in a stable patient whose rupture was limited to mediastinum and sepsis had not developed [8]. In our patient in retrospect, CT chest from 2 weeks prior to transplant showed some concern regarding esophageal compromise but was unfortunately missed. The current large perforation therefore was due to instrumentation and microleak, which over time likely progressed. In several reports, cases treated with nonoperative management involved patients with perforation smaller than 11 mm in size, while surgery was recommended for perforations larger than 2.5 cm [8]. Given the long duration from her initial insult and clinical stability, we opted to utilize a previously reported approach of transesophageal drainage of contained esophageal perforations [9]. We reserved surgical therapy for any clinical deterioration, or if the transesophageal drainage approach failed to heal the perforation. Vogel et al. [10] presented their experience with 47 patients with esophageal perforation confirming the success of aggressive nonoperative approach to iatrogenic and spontaneous esophageal disruptions, with overall survival of 96%. Of the 32 patients treated nonoperatively, there was 100% survival. The approach involved aggressive drainage of fluid collections and frequent CT and gastrografin upper gastrointestinal series exams to evaluate progress.
We found that by day 16 (d16) postdiagnosis of perforation, we were able to start pulling back on the tube. Further pulling back of the NP was dictated by improvement we saw on subsequent esophagograms. By d30, NP was removed and follow-up of esophagogram on d33 showed no extravasation of the contrast, suggesting healed perforation. She was started on sips of water and diet slowly advanced with soft regular diet 2 weeks postdischarge (Figs 2 and 3).
In conclusion, we utilized a simple nonoperative means to successfully manage a delayed large esophageal perforation in a stable immunocompromised patient who underwent a complicated liver transplant operation. Aggressive treatment of sepsis, pleural fluid collections with frequent radiologic confirmation, along with NPO, enteric nutrition, antimicrobials and antifungals as well as judicious scaling back on immunosuppression form an important aspect of the treatment strategy.



